Sweet secrets analyzed fast and easily
Automated validation procedure of an optimized UHPLC assay
With today’s growing health awareness, there is an increasing interest in sugar substitutes in various areas of the food industry. Compared to well established artificial sweeteners, such as Aspartam and Saccharin, the natural alternative Stevia, extracted from the Stevia rebaudiana plant has been approved for use in the EU only in 2011. Stevia, also known as sweet-leaf, is native to South America and the plant extract contains steviol glycosides with up to 450 times the sweetness of sugar.
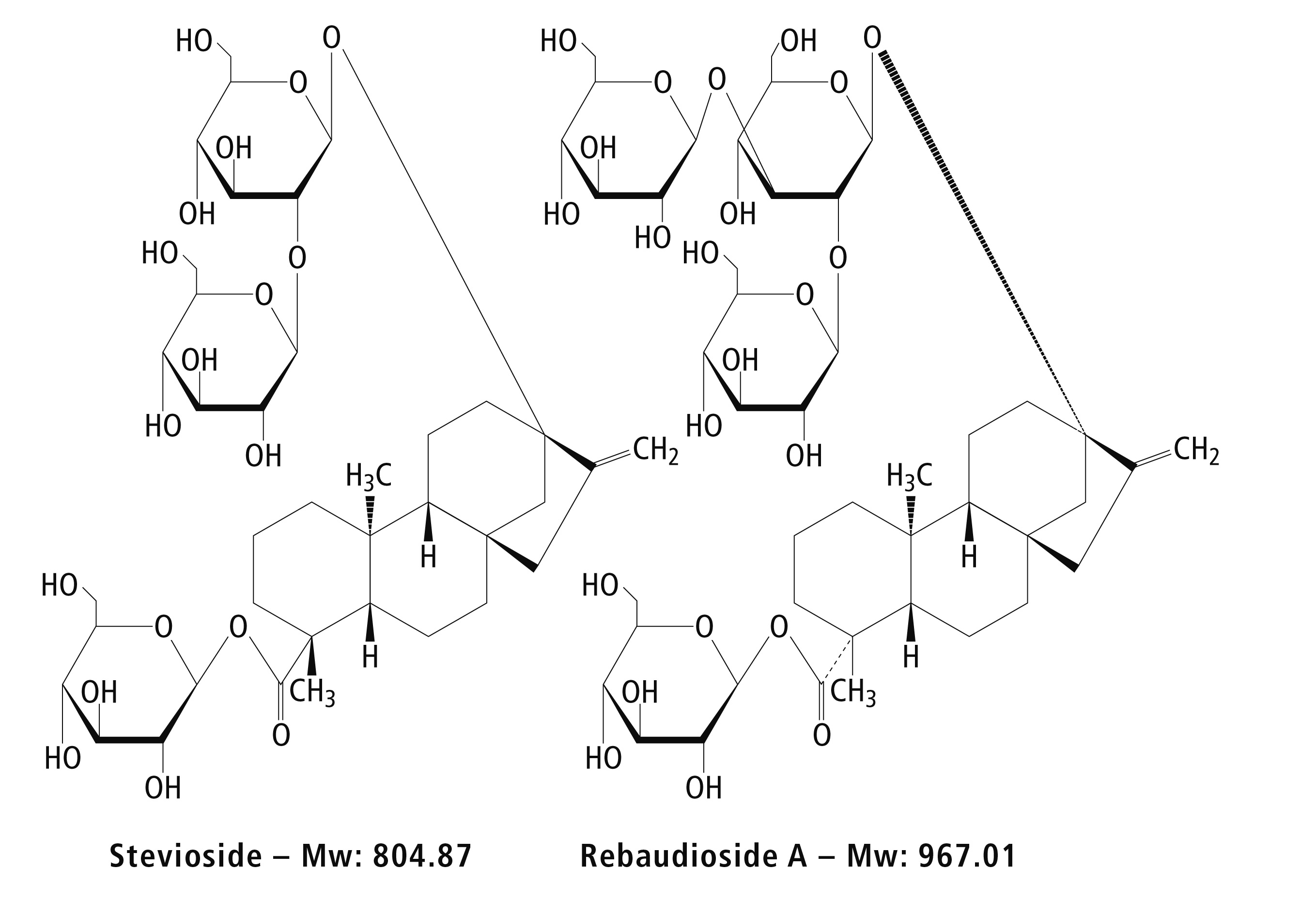 Figure 1: Structures of stevia glycosides
Figure 1: Structures of stevia glycosides
As the Rebaudioside A steviol glycoside exhibits the lowest bitter aftertaste, it is largely enriched in commercial stevia products, while natural extract contains stevioside as the major component. Hence, an HPLC assay for quality control purposes, must be able to clearly separate this critical peak pair. After method development using the Nexera X2 Method Scouting Platform, the Nexera-i new generation integrated UHPLC system with its system check function and automated workflow was the ideal tool for a quick and simple programmed method validation procedure.
Starting point for the development of an improved UHPLC method for the separation and quantification of nine stevia glycosides was the JECFA (Joint FAO/WHO Expert Committee on Food Additives) approved method, routinely used for the quality assurance of stevia analytical standards.
Method Development
JECFA method (Figure 2 a):
Column: Phenomenex Luna C18(2) 5 µm; 250 x 4.6 mm
Mobile Phase: A: 10 mM NaH2PO4, pH 2.6 in H2O to B: ACN (68:32 v/v)
Isocratic: 35 min run time
Flow rate: 1 mL/min
Temperature: 40 °C
Injection Volume: 5 µL
Sample: 0.2 mg/mL of each compound in MeCN/H2O (30:70 v/v)
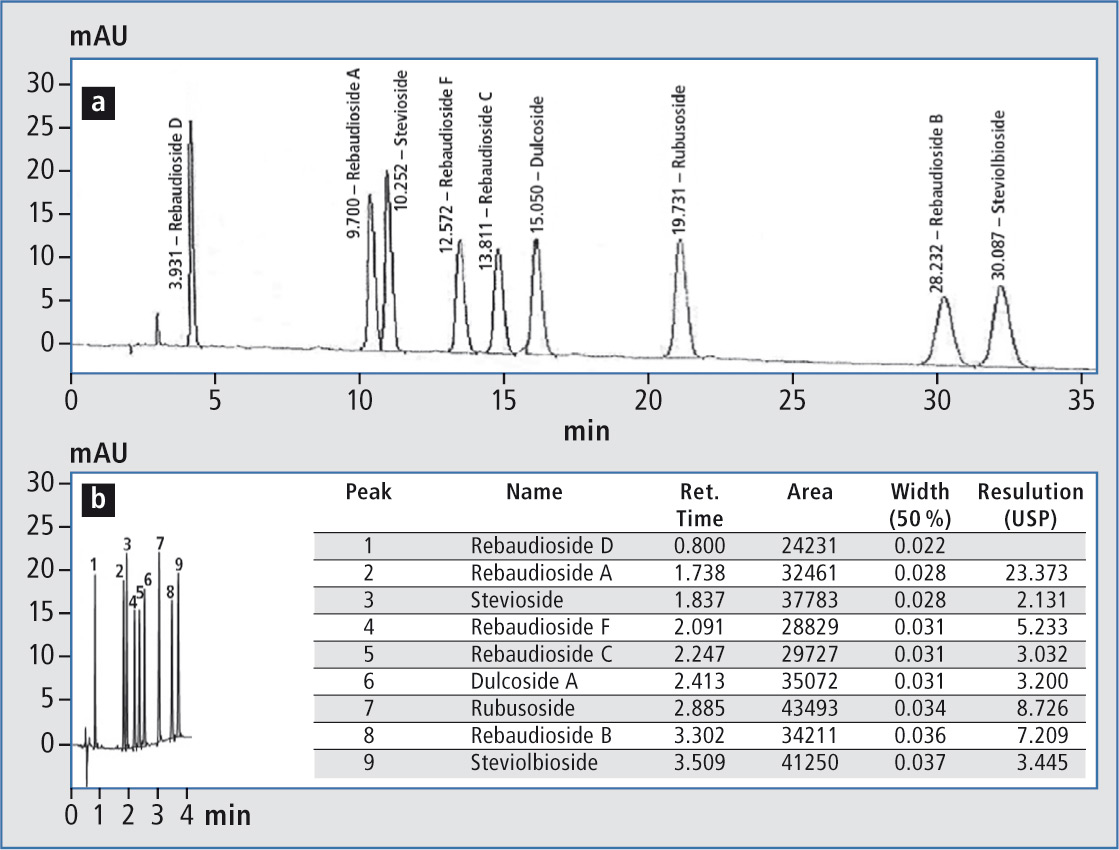 Figure 2: a) JECFA method for the separation of nine stevia glycosides b) improved UHPLC assay for the separation of nine stevia glycosides
Figure 2: a) JECFA method for the separation of nine stevia glycosides b) improved UHPLC assay for the separation of nine stevia glycosides
For UHPLC method scouting, a Shimadzu Nexera X2 Method Scouting System was used, consisiting of two quaternary solvent pumps, an autosampler and a column oven including a six column switching valve. The system was also equipped with a photo diode array detector (Figure 3).
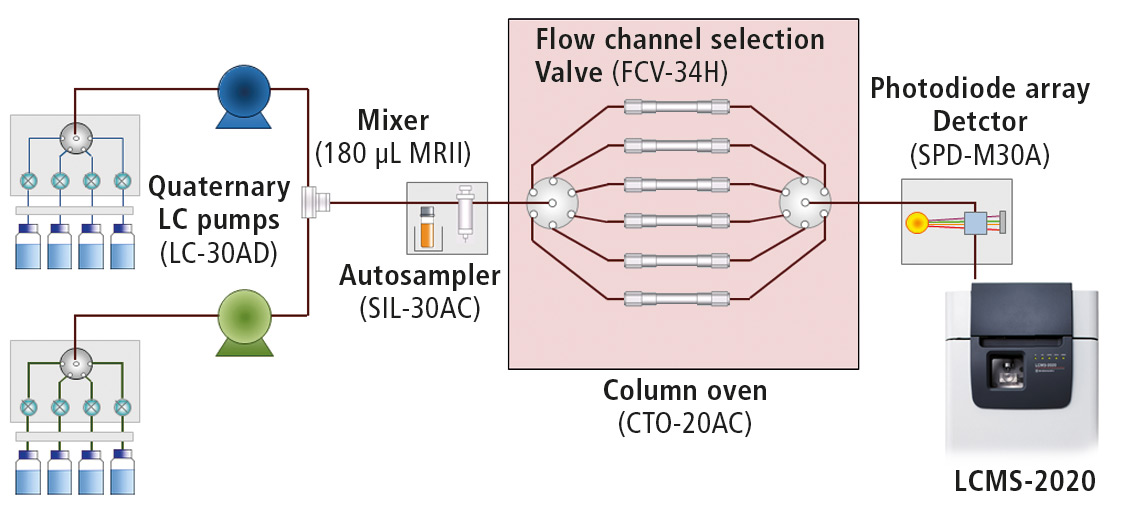 Figure 3: Schematic diagram of the Nexera X2 Method Scouting System
Figure 3: Schematic diagram of the Nexera X2 Method Scouting System
Method scouting was performed in an overnight sequence using 3 and 9 min gradient runs at 30 °C and 50 °C. Five combinations of stationary phase and mobile phase were selected. The data obtained from the most promising mobile phase/stationary phase combination was used for computer simulation to identify the optimum separation conditions with respect to gradient slope and oven temperature.
Resulting UHPLC method (figure 2 b)
Column: ACE Excel 2 Super C18, 150 x 2.1 mm
Mobile Phase: 10 mM NaH2PO4, pH 2.8 A: in H2O and B: in MeCN/H2O (80:20 v/v)
Gradient: 39.5 – 48 % B in 4 min
Cycle time: 7 min
Flow rate: 0.6 mL/min
Temperature: 50 °C
Injection Volume: 1 µL
Sample: 0.04 mg/mL of each compound in MeCN/H2O (6:94)
Validation
The method obtained was fully validated using the LabSolutions / VALIDAT interface. The incorporated template assistant required merely the addition of the appropriate components to be validated, which was achieved by importing the LabSolutions method file (.lcm) with compound table into the VALIDAT project (Figure 4).
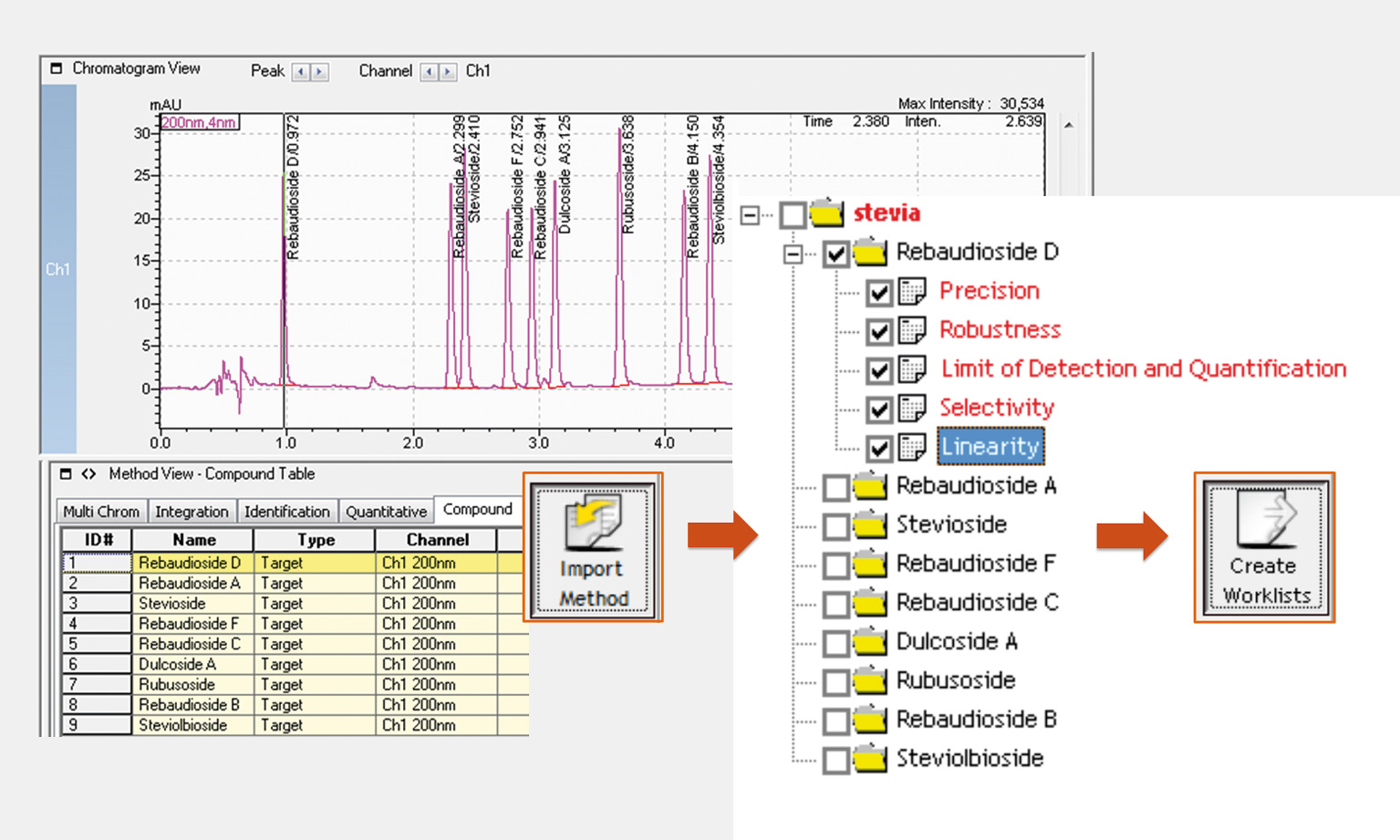 Figure 4: Validation planning by method import from LabSolutions into VALIDAT©
Figure 4: Validation planning by method import from LabSolutions into VALIDAT©
To carry out the validation successfully, the software provided all mathematical and statistical procedures, as well as full 21 CFR Part 11 compliance. With a well-structured workflow and versatile, adjustable templates the validation process was organized easily and efficiently (Figure 5).
 Figure 5: Method validation workflow guided by VALIDAT©
Figure 5: Method validation workflow guided by VALIDAT©
After importing the method, each parameter, such as precision, linearity, repeatability etc. was planned individually. According to these checkpoints VALIDAT released a validation plan as a starting point for programmed batch creation in LabSolutions. By importing the batch from VALIDAT into LabSolutions each data file (.lcd) obtained a unique identifier that allowed automated export of the analytical results back into the validation software, eliminating the need for manual copy-pasting and the risk of human error during this process.
Additionally, the i-series ‘Auto Validation’ function (Figure 6) allows to easily evaluate whether or not the system is working in a stable range. System parameters, such as solvent delivery or wavelength accuracy are controlled by a fixed procedure to guarantee the system performance before carrying out a method validation.
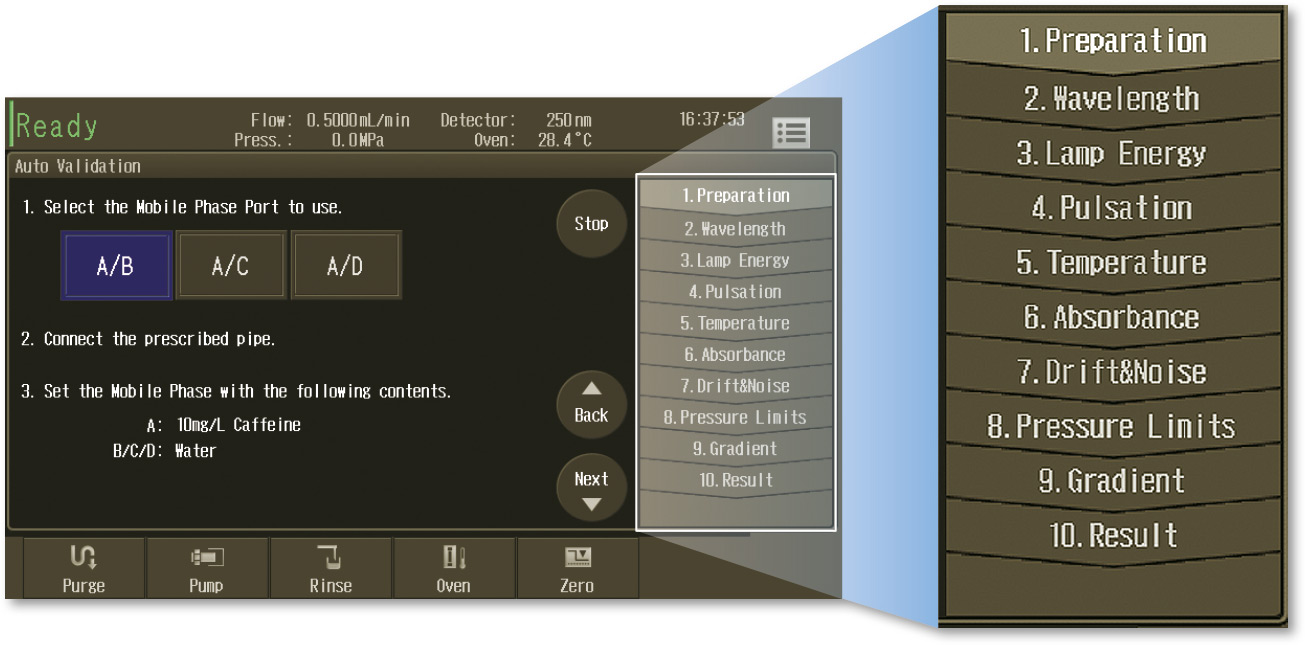 Figure 6: Auto-validation procedure in i-series instrument
Figure 6: Auto-validation procedure in i-series instrument
It is also possible to carry out an automated routine inspection and self-diagnosis of the instrument via the ‘System Check’ function, where data on consumable usage is recorded, such as number of injections, solvent volume delivered by the pump or UV lamp burn time. The results of the auto validation and system check procedures are summarized in a printed report that can be added to the validation documents.
After the system status had been evaluated, the LabSolutions batch file allowed full automation of the sample analysis from starting of the instrument to the shut-down procedure after the run had finished.
All necessary steps, such as switching on the instrument at a defined time, purging of the flow lines, column/system equilibration until a stable baseline was obtained, sample measurements, system wash and shut-down were programmed into the batch table and carried out automatically in an overnight run (Figure 7).
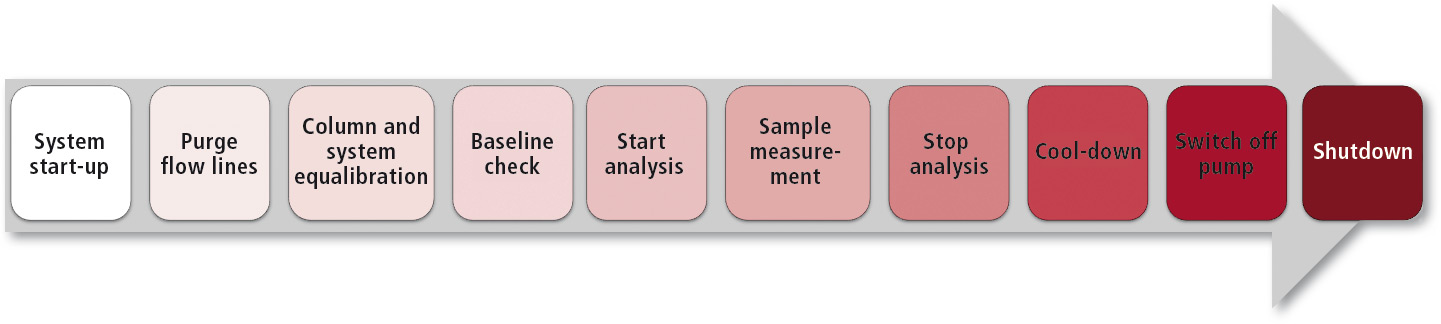 Figure 7: Automated workflow in i-series batch analysis
Figure 7: Automated workflow in i-series batch analysis
Results
After electronic release of the validation plan for programmed batch creation in LabSolutions and analysis on the i-series using the workflow described in figures 4 – 7, the pre-defined validation report (Figure 8) was completed within minutes thanks to the automated data import into VALIDAT. The successfully completed validation project was then saved as a template that will serve as a starting point for further projects.
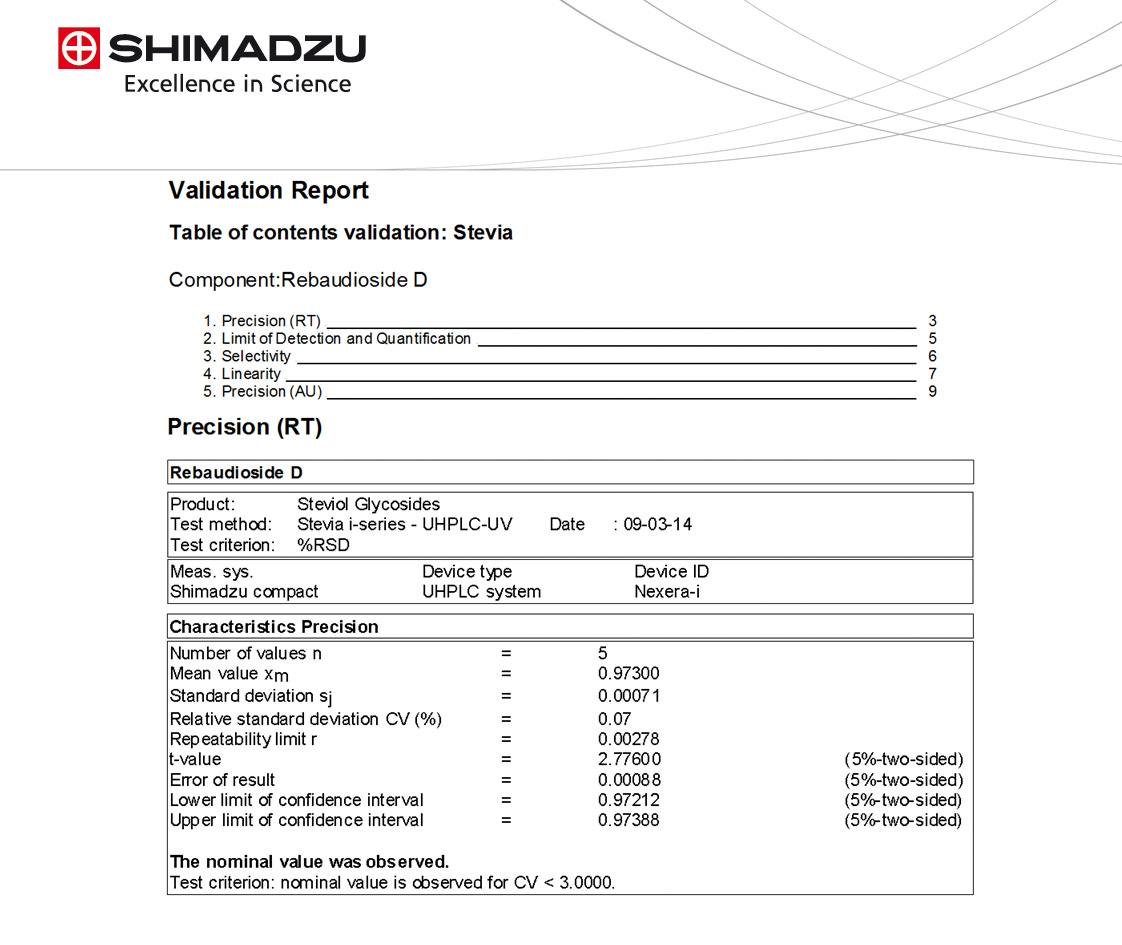 Figure 8: Finished validation report created by VALIDAT© according to company template VA
Figure 8: Finished validation report created by VALIDAT© according to company template VA