New generation of protein sequencer
PPSQ-50 with increased sensitivity and FDA compliance
Protein sequencing via Edman degradation is a proven and still very popular method in many laboratories. With the new PPSQ-50 protein sequencer series, Shimadzu has now further developed the successful PPSQ series. Popular features like robustness, reproducibility and low running costs have been retained while sensitivity has been increased significantly by using a far more sensitive detector.
In addition to its customary simplicity of use, the new LabSolutions PPSQ software for instrument operation and data evaluation now also offers optional compliance with the 21 CFR Part 11 guidelines of the US Food and Drug Administration (FDA). Two versions of the sequencer are available:
- the PPSQ-51A equipped with one reactor
- the PPSQ-53A with three reactors enabling simultaneous placement of up to three samples into the instrument with subsequent sequential processing. This ensures the best possible system utilization, for instance during the night or over the weekend.
Sequencing according to Edman degradation
In the main unit of the PPSQ, the N-terminal amino acids of a protein or a peptide which is immobilized on a membrane are derivatized and cleaved off via the Edman degradation reaction.
The remaining protein or peptide is available for the next cycle so that, little by little, all N-terminal amino acids are analyzed.
Meanwhile, the PTH amino acids are injected into the HPLC unit where they are identified chromatographically via the specific retention times of each amino acid and comparison with a standard. Because the separation takes place isocratically on a C18 column, the mobile phase can be continuously recycled and reused. This not only reduces purchase and disposal costs of the eluent, but also enables quick startup, even when the system has not been used for longer periods since gradient optimization is not necessary. Another advantage of isocratic analysis is the baseline and retention time stability.
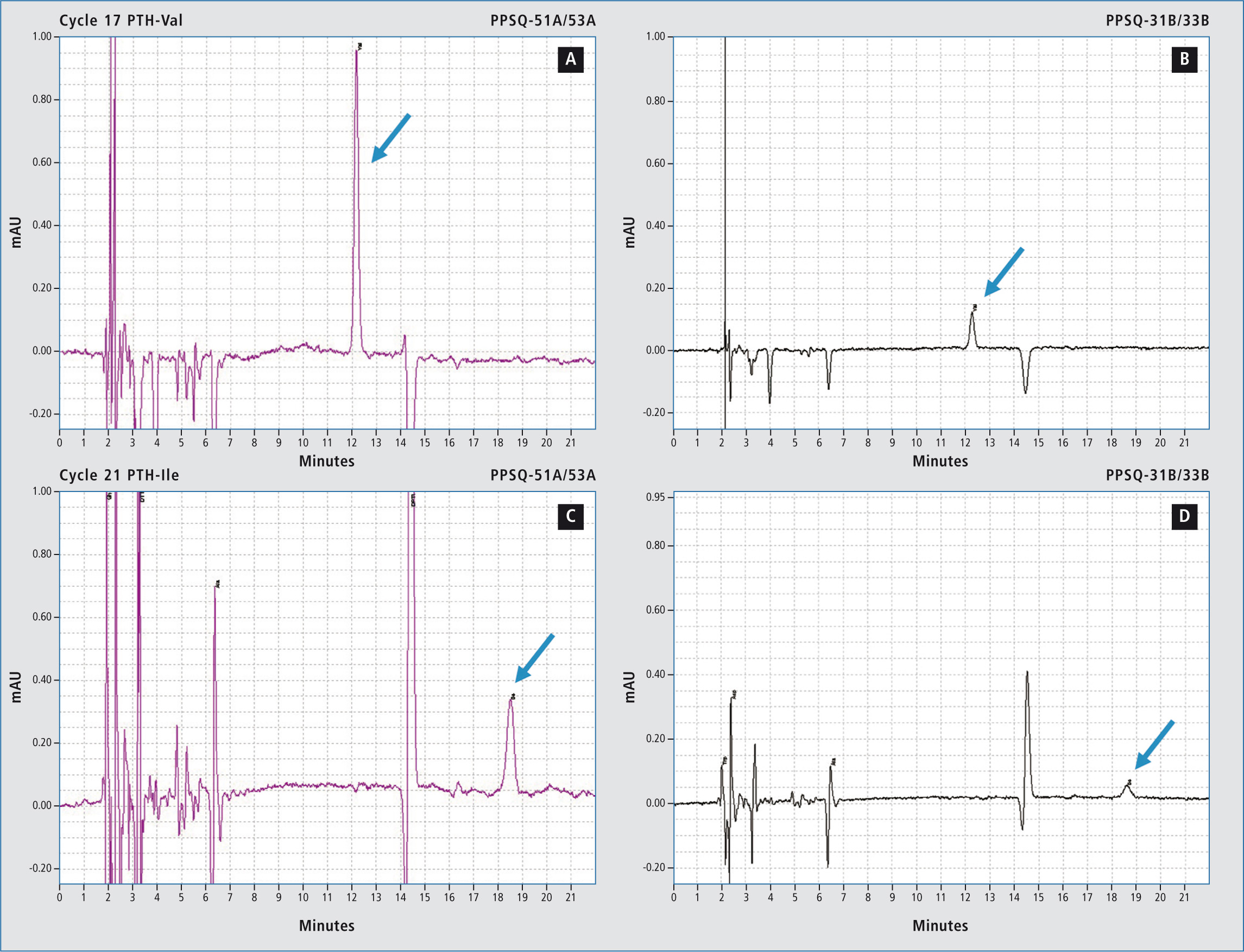 Figure 1: Sample: Horse myoglobin 10 pmol; results show the subtraction chromatograms by PPSQ-50 (A, C) and PPSQ-30 (B, D) connected in tandem after Edman degradation
Figure 1: Sample: Horse myoglobin 10 pmol; results show the subtraction chromatograms by PPSQ-50 (A, C) and PPSQ-30 (B, D) connected in tandem after Edman degradation
Increased sensitivity – also upgradable
The design of the new PPSQ-50 (figure 2) is more compact than the predecessor series and requires less laboratory bench space. In addition, the new detector achieves a significantly higher detection sensitivity in comparison with the PPSQ-30 series.
Figure 2 shows a tandem analysis in which the sample was analyzed using the new detector as well as that of the predecessor model. It is easy to see that detection via the new unit is far more sensitive. In this way, it is possible to determine long protein sequences, even for samples of which only small amounts are available. As the separation column, the eluent and the analytical conditions are still the same, it is possible to upgrade previously acquired instruments of the PPSQ-30 series and thereby also profit from the increased sensitivity.
 Figure 2: More compact design of the PPSQ-50 series with reduced footprint
Figure 2: More compact design of the PPSQ-50 series with reduced footprint
Software solutions for regulated environments
The newly developed LabSolutions PPSQ software incorporates instrument operation as well as data evaluation. In addition to automated sequence determination, the user-friendly software also enables reprocessing and overlaying of chromatograms, as well as a subtraction mode that shows differential chromatograms of two sequential ones for simple and fast sequence determination.
The LabSolutions PPSQ software is available in three versions: The classic version (LabSolutions PPSQ) and a version with integrated database (LabSolutions PPSQ DB), which are both suitable for use in stand-alone instruments. A new feature is the possibility to work in accordance with the 21 CFR Part 11 guidelines of the FDA. This includes security settings, user administration, history documentation (audit trail), as well as data storage in a database.
The third version is a client/server solution (LabSolutions CS) that allows network integration of the instrument so that data evaluation can be carried out from any computer. Clients already using a PPSQ-30 system can easily switch to this new software, which makes working in a regulated environment much easier thanks to the compliance achieved.