Higher performance with Atomic Booster
WizAArd software and Atomic Booster optimize AA-7000F sensitivity
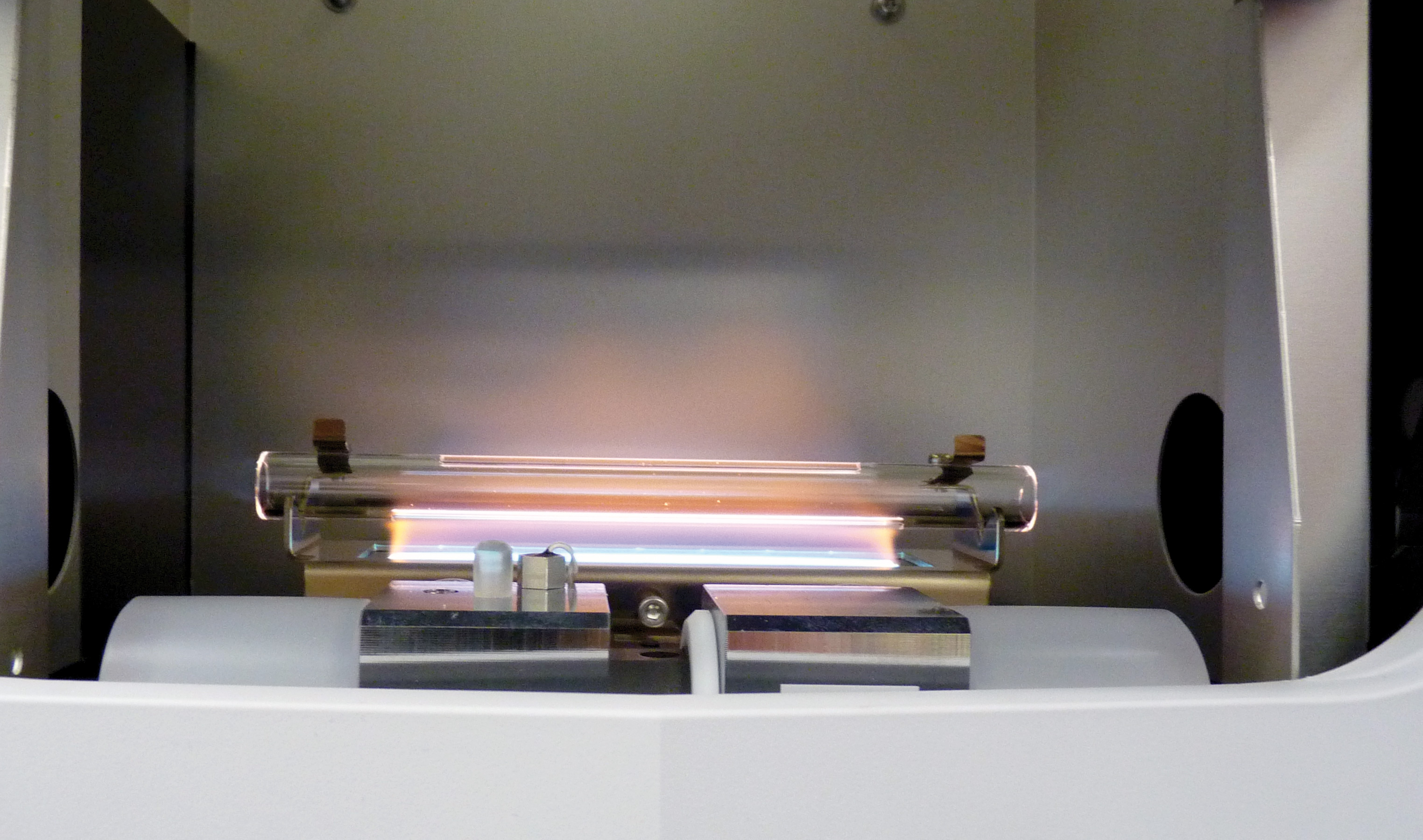
Flame AAS is one of the fastest methods in elemental analysis. However, sensitivity of the measuring method often plays a decisive role. How can sensitivity be optimized? This article presents an overview of various optimization approaches using the AA-7000F atomic absorption spectrophotometer.
In order to optimize sensitivity, the flame gas composition as well as flame observation height can be adjusted. These instrument setting parameters are adjusted individually as part of an automated optimization process using the associated WizAArd software.
Fast method creation using the WizAArd software
The WizAArd software provides complete instrument control. Basic parameters for the respective elements are also stored here. In this way, new methods can be created very effectively and require very little time.
Because of the flexibility of the analytical instrument, it is particularly easy to optimize the burner height. It is possible to carry out measurements not only in the flame mode but also in the much more sensitive graphite furnace mode. This option can also be implemented afterwards.
A software-controlled motor enables hands-free switching between flame and furnace mode (AAC: Automatic Atomizer Changer). This motor is also used to adjust the burner height in the flame mode when not using the graphite tube furnace. Flame gas composition can also be adjusted fully automatically via the software.
Optimization of the burner height
In flame AAS, the liquid sample is first nebulized and subsequently desolvated by the thermal energy (temperature) of the flame, and the elements (present in the flame as ionic or molecular species) are further atomized into free atoms. Only in this way the measuring principle does fully apply.
Element-specific light is passed through the flame. The flame acts as a measuring cell, similar to the cuvette used in UV-VIS spectroscopy. The elements present in the flame can now absorb the element-specific light. The more atoms are present, the stronger the attenuation of the light. In addition to qualitative element identification, quantitative measurements can also be carried out.
Depending on the characteristics of the element being investigated, the atoms are present at different heights (temperature zones) in the flame. If the flame observation height (height within the flame where absorbance is measured) is too low, the elements have not yet been atomized and cannot be detected correctly. If the flame observation height is too high, the elements may be present in their excited/ionized state, whereby they are no longer detected.
The sample matrix can have an additional effect on the flame. If, for instance, the sample contains organic compounds such as ethanol or methanol, the flame is generally hotter. This means that the flame must be observed at a lower height, or that the flow rate of the combustion gas must be minimized.
Example cesium
The element cesium (Cs) is used to optimize the burner height. Cs is quite sensitive to atomic absorption with limits of detection in the double-digit ppb range. In this way, it outperforms ICP-OES (single-digit ppm range). The determination of even lower Cs levels is possible using graphite furnace AAS (double-digit ppt range).
To optimize the burner height, an interval is selected within which various burner heights are tested under simultaneous nebulization of a Cs standard solution. Measuring values are subsequently determined (figure 1a). In this case, the lowest observation height is the optimal choice because Cs is thermally very unstable. Cs atomization already takes place very close to the burner head.
Optimization of the flame gas composition
Two different gas mixtures are mainly used for the flame. In both cases, acetylene (C2H2) is the flame gas. Different so-called oxidants are used: air for low flame temperatures and nitrous oxide (N2O) for higher flame temperatures. For most elements, air will be sufficient to generate free atoms in the flame. For refractory elements with high dissociation energies, higher flame temperatures are required (for instance aluminum or titanium).
Air-acetylene flames are selected for Cs. This is also stored in the software. Analogous to the optimization of the burner height, an interval is selected for the flame gas flow (acetylene). The resulting plot of absorbance versus flow rate shows a signal maximum at a flow rate of 2.4 L/min (figure 1). This parameter is now stored as the optimum flow rate in the method.
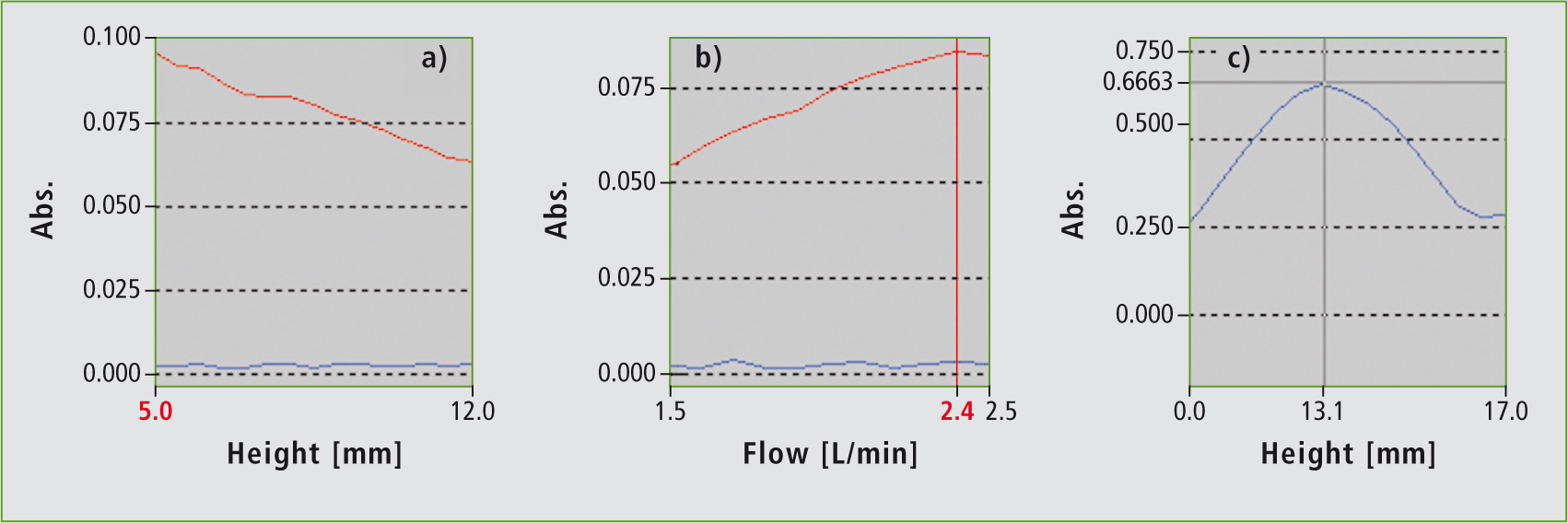 Table 1: AA-7000F – Limits of detection of cesium and cadmium before and after individual optimizations (calculated according to DIN 32645). The corresponding calibrations are shown in figure 2.
Table 1: AA-7000F – Limits of detection of cesium and cadmium before and after individual optimizations (calculated according to DIN 32645). The corresponding calibrations are shown in figure 2.
Effects on the detection limit: cesium
Not only the sensitivity, i.e. the highest measuring signal, but also signal fluctuations have an effect on the detection limit. For this reason, improvement of the detection limit is determined via the linearity of the calibration (DIN 32645). Comparing the values (table 1), it becomes clear that the detection limit is already significantly improved by adjusting the flame gas composition. Adjustment of the burner height brought about further improvement of the detection limit so that it was possible in standard flame operation to detect concentrations as low as 0.011 mg/L. The effects of the individual steps on the calibration of cesium are shown in figure 1 (a – c).
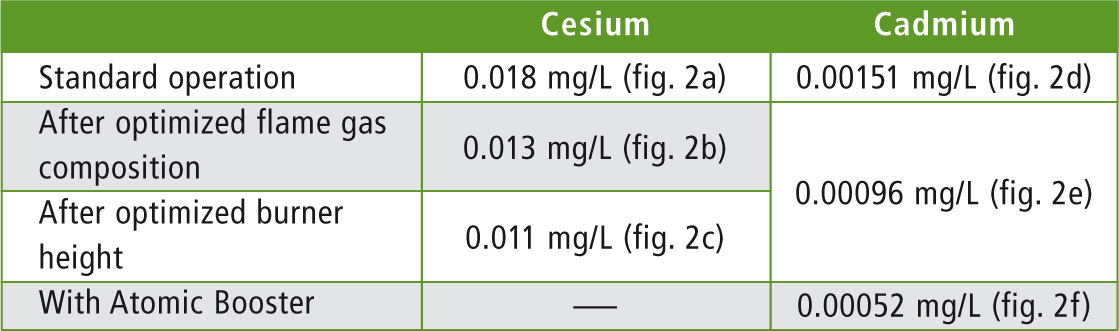 Figure 1: a) Cs – Optimization of burner height; b) Cs – Optimization of flame gas composition; c) Cd Atomic Booster – Optimization of burner height
Figure 1: a) Cs – Optimization of burner height; b) Cs – Optimization of flame gas composition; c) Cd Atomic Booster – Optimization of burner height
Atomic Booster – optimization for cadmium
As an alternative to cesium, Cadmium was used for optimization. Cadmium is a heavy metal and is toxicologically very harmful. It is already considered poisonous at low concentrations and has been proven to be carcinogenic as well as mutagenic and teratogenic. For humans it is not required physiologically (non-essential element). This is why trace analysis of this element is of critical importance.
To improve the detection limit of the AA-7000F for this element, the flame gas composition and the burner height were optimized in the same way as for cesium, leading to calibrations with differing sensitivities (see figure 2 d, e). The detection limit for cadmium could also be lowered using these optimization steps. Instead of 1.5 µg/L, a detection limit of 1.0 µg/L could be achieved (table 1). To further increase the detection sensitivity for this toxicologically relevant element, the Atomic Booster is used.
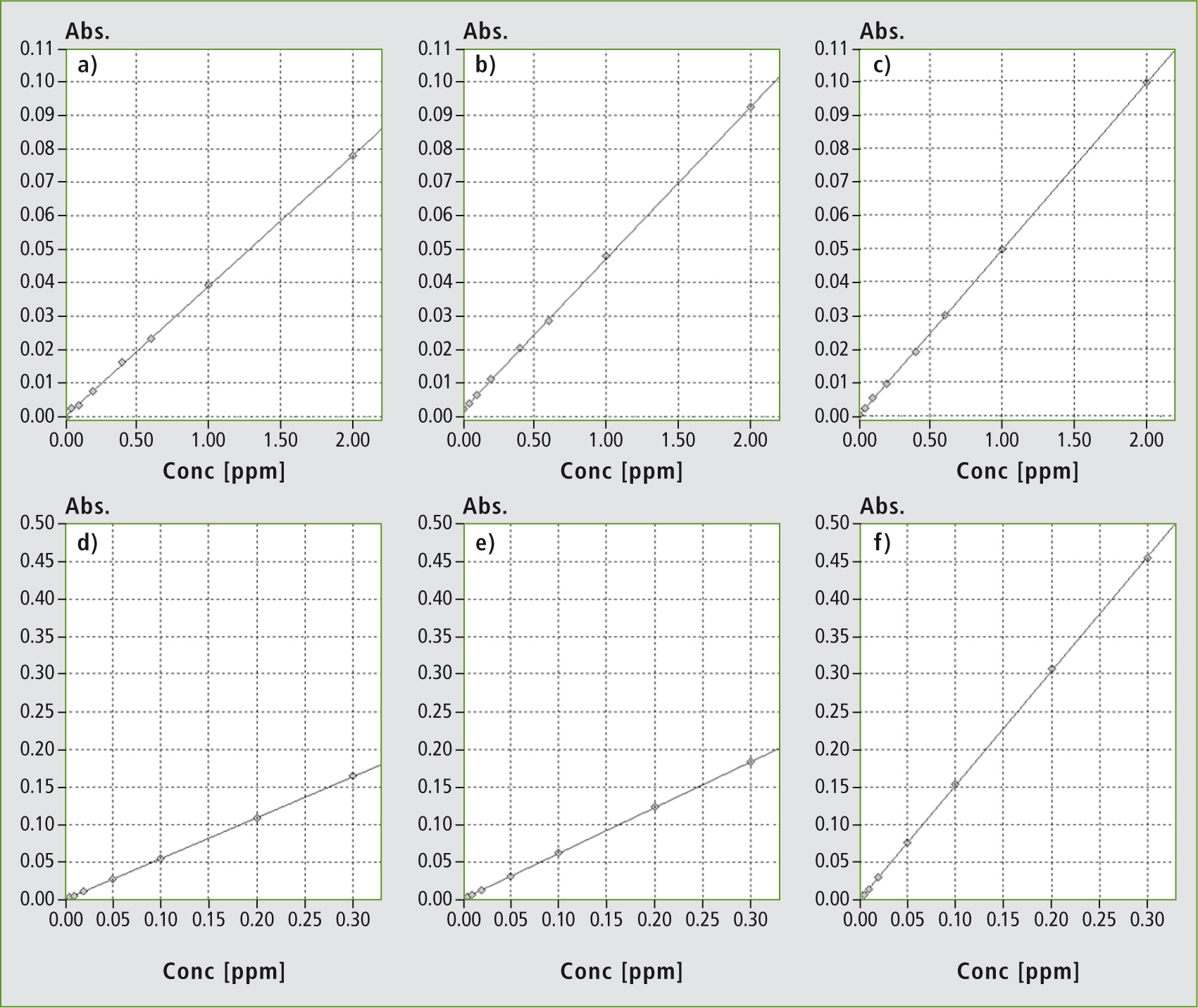 Figure 2: Calibration of cesium and cadmium before and after individual optimizations. Details are provided in table 1
Figure 2: Calibration of cesium and cadmium before and after individual optimizations. Details are provided in table 1
The Atomic Booster is essentially a quartz tube that is positioned in the optical path over the exit slot of the burner. The tube has two slit-shaped openings opposite each other that are different in length. The flame enters the quartz tube through the longer (larger) opening. Parts of the flame can subsequently leave the quartz tube through the shorter opening. However, since the exit opening is smaller, a portion of the flame is held within the optical path or leaves the quartz tube through both open ends (figure 3). In this way, the dwell time of the atoms in the optical path as well as the ‘thickness’ of the flame can be increased.
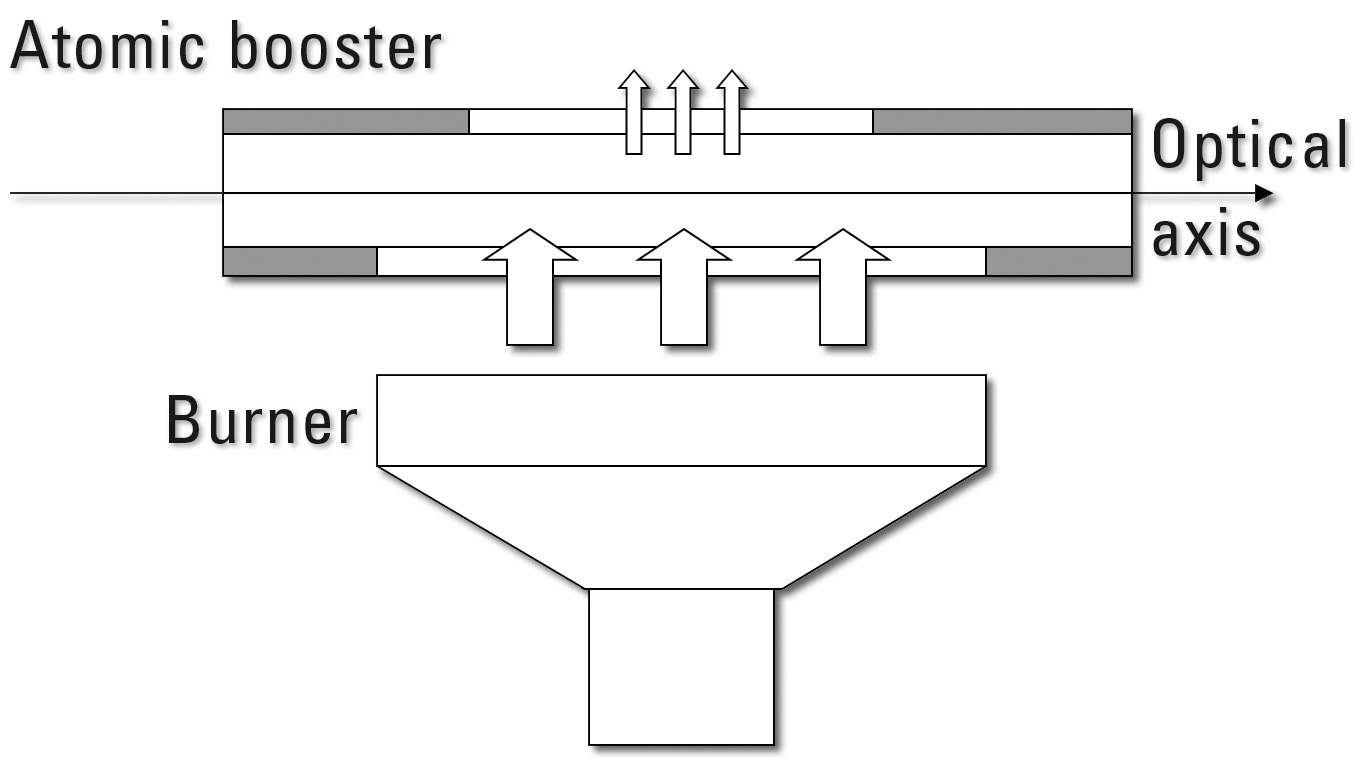 Figure 3: Principle of the Atomic Booster – Dwell time of the atoms in the optical axis is increased
Figure 3: Principle of the Atomic Booster – Dwell time of the atoms in the optical axis is increased
To achieve a maximum energy throughput of the element-specific light (good positioning within the optical axis), the burner height must also be adjusted correctly. The lamp mode is set to emission and the maximum burner height is determined (figure 1c). Measurements using the Atomic Booster take place at a burner height of 13 mm.
By focusing the flame in the optical axis, sensitivity increases significantly, as can be seen in the calibration plot (figure 2f) in comparison to measurements without Atomic Booster (figure 2 d, e). This increase in sensitivity lowers in turn the detection limit by a factor of 2, whereby the detection limit for flame AAS lies within the ppt range (0.52 µg/L).
Conclusion
Using different approaches, the instrument sensitivity of the AA-7000F can be adjusted quickly and easily and optimized to the sample matrix at hand. The supporting functions of the WizAArd software enable automated optimization of burner height and flame gas composition. Detection limits of 1 µg/L (for cadmium) can be achieved in this way. Cesium is also very sensitive with a detection limit of 11 µg/L.
Using the Atomic Booster, further sensitivity increases can be achieved. The principle is quite clear and the results speak for themselves: the detection limit is halved and at 520 ng/L and lies within the ppt range.