Mineral oil compounds in paper and cardboard packaging
Investigating the composition of the aromatic mineral oil fraction
The presence of saturated and aromatic mineral oil hydrocarbons (MOSH – Mineral Oil Saturated Hydrocarbons and MOAH – Mineral Oil Aromatic Hydrocarbons) in paper and cardboard packaging for food and hygiene products is an issue that is being widely discussed at the moment. The MOSH fraction consists of linear and branched alkanes as well as alkyl-substituted cycloalkanes, whereas the MOAH fraction consists of alkylated polyaromatic hydrocarbons with up to four aromatic rings. The main focus is on the aromatic fraction, as it may contain potential carcinogenic and mutagenic compounds [1]. The proportion of the aromatic fraction is approximately 15 – 30 % of the total mineral oil fraction. The main sources of mineral oil compounds in paper and cardboard are printing inks, which are either applied directly onto the packaging or introduced via the recycling processes during which they are not fully removed.
The migration of mineral oil hydrocarbons from packaging into packaged products takes place through direct contact or via the gas phase, by evaporation from the packaging and re-condensation in the packed product. These processes can result in a relevant migration of mineral oil compounds with carbon chain lengths of up to n-C28.
Mineral oil fraction migrates into packed products
The concentration of mineral oil hydrocarbons ≤ n-C28 in paper products is up to 1000 mg/kg [2], of which up to 70 % can migrate into the packed product. Based on the acceptable daily intake of 0.01 mg/kg body weight, as specified by the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) in 2002, a specific migration limit for MOSH of 0.6 mg/kg and for MOAH of 0.15 mg/kg packed product has been defined under assumptions of standard conditions.
At present, analytical determination of MOSH and MOAH is mainly carried out via online or offline high-performance liquid chromatography coupled with gas chromatography-flame ionization detection and large-volume injection (HPLC-GC-LVI-FID) [4]. Concentration of MOSH and MOAH is determined in this way.
Large-volume injection (LVI) with flame ionization detection (FID) is used to achieve sufficient sensitivity and to avoid detector discrimination. This, however, causes fractions to elute as non-separated ‘mineral oil humps’ (broad peaks) and identification of individual compounds is not possible (figure 1). Because of this lack of detailed information, it is difficult to evaluate the mutagenic potential of the MOAH fraction. For this reason, an established evaluation method is at present not available.
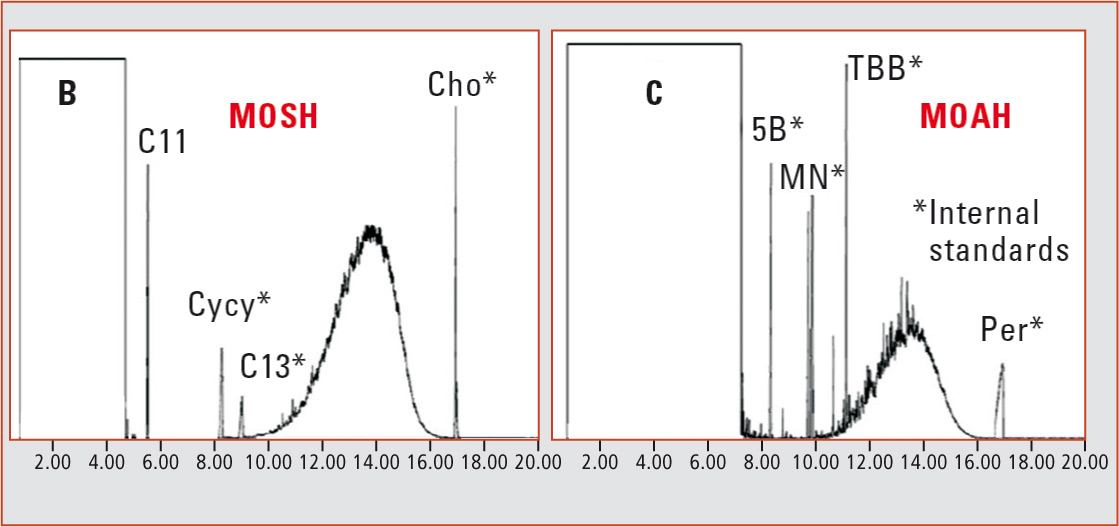 Figure 1: GC-FID chromatogram of a MOSH and MOAH fraction [5]
Figure 1: GC-FID chromatogram of a MOSH and MOAH fraction [5]
Multidimensional GC increases sensitivity and selectivity
Gas chromatography with a mass selective detector offers one possibility to obtain detailed information on the composition of the MOAH fraction. In particular, the so-called comprehensive GCxGC separation is the technology of choice for increased sensitivity and selectivity.
Are newspapers the source of printing ink migration into packaged products?
Newspapers and magazines are part of the raw materials for the production of recycling products for many different applications. The mineral oil containing printing inks are considered to be the main sources of contamination in recycling products. As starting material, various daily newspapers were analyzed. In addition, different packaging papers (fresh fiber paper and recycling cardboard) were analyzed.
Sample preparation was carried out by extracting the paper samples and separation of MOSH and MOAH via HPLC. Subsequently, GCxGC-MS analysis of the MOAH fraction was executed using Shimadzu’s GCMS-QP2010 Ultra with a Zoex cryogenic modulator. Details of the analytical parameters are shown in table 1.
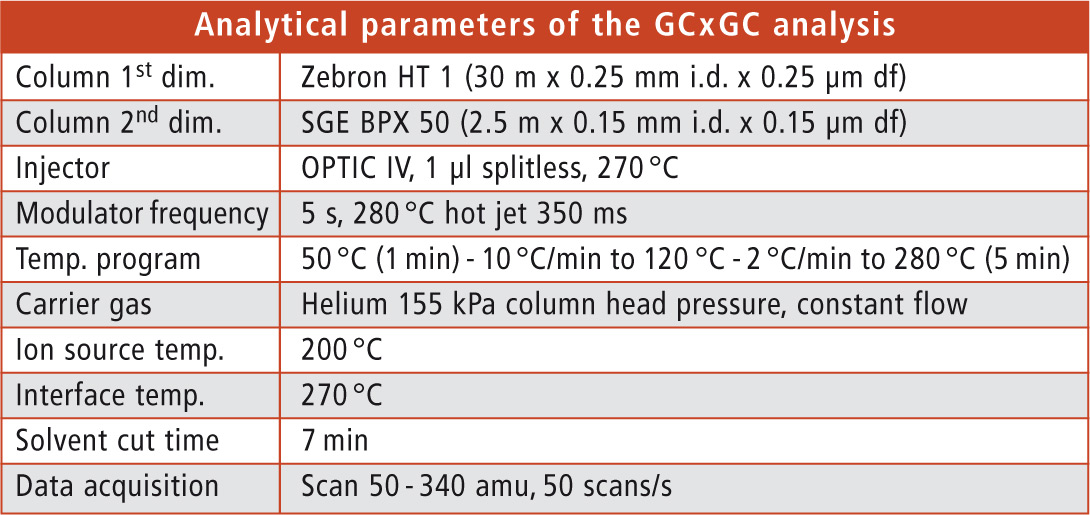 Table 1: Analytical parameters of the GCxGC analysis
Table 1: Analytical parameters of the GCxGC analysis
For identification of individual compounds, a standard mix consisting of the internal standards of the MOSH/MOAH separation [2, 5], alkylated aromates and polyaromatic hydrocarbons (PAHs) as non-alkylated marker substances was used.
GCxGC-MS analysis identifies phenylalkanes as dominating aromatic substance group
Using multidimensional GCxGC-MS analysis, it was possible to identify linear and branched phenylalkanes as the dominating aromatic substance group. An unequivocal identification of the phenylalkanes was carried out using a standard mix containing linear phenylalkanes with a carbon chain length of C5 to C18 (figure 2).
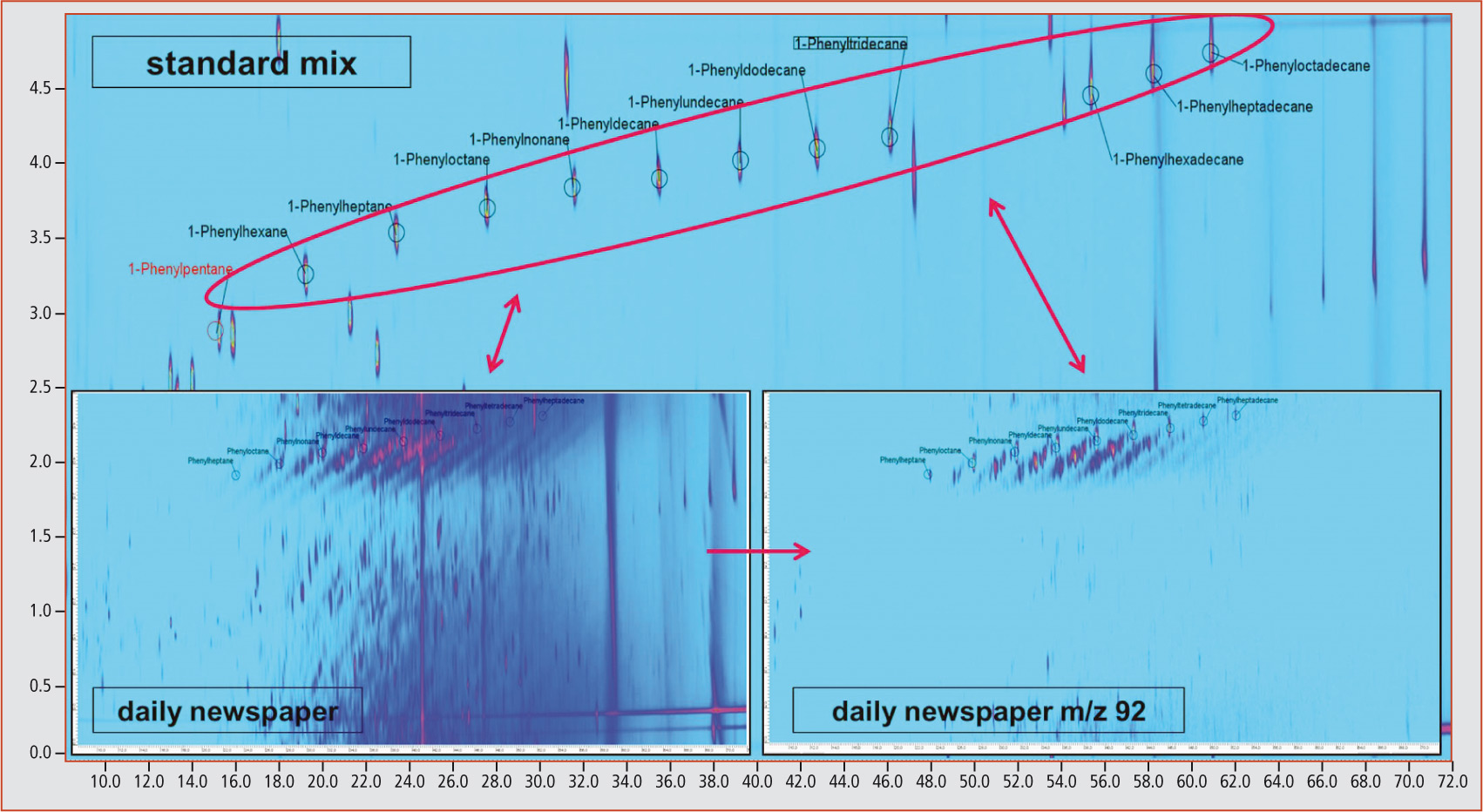 Figure 2: Phenylalkanes (Scan and m/z 92) in standard and sample (newspaper)
Figure 2: Phenylalkanes (Scan and m/z 92) in standard and sample (newspaper)
Through extraction of the mass fragment m/z 92, characteristic for the phenylalkanes, diagonal lines of unknown compounds become apparent that are also characterized by the mass fragment m/z 92 originating from the linear phenylalkanes. The exact analysis of these unknown compounds revealed that all compounds lying on one diagonal originated from the same mass fragment as the linear marker substance.
This is illustrated for phenylundecane isomers in figure 3. Through extraction of the m/z 92 fraction, it is possible to recognize the diagonals originating from the linear phenylalkanes. By extracting the mass fragment m/z 232 that is typical for phenylundecane, it becomes apparent that all compounds on a diagonal are characterized by the same molecular mass. An identical molecular mass points towards differently substituted and branched analogs. This could also be shown by verification of the compounds 1-phenylpentane, isopentylbenzene and (1-methylbutyl)benzene contained in the standard mix (figure 4). Extraction of the characteristic molecular mass fragment m/z 148 revealed that these three compounds are on a straight line (dotted line). An unequivocal identification of the unknown differently substituted and branched analogs of linear phenylalkanes with longer carbon chains is, however, currently not possible due to a lack of available standards. All samples were contaminated with mineral oil components
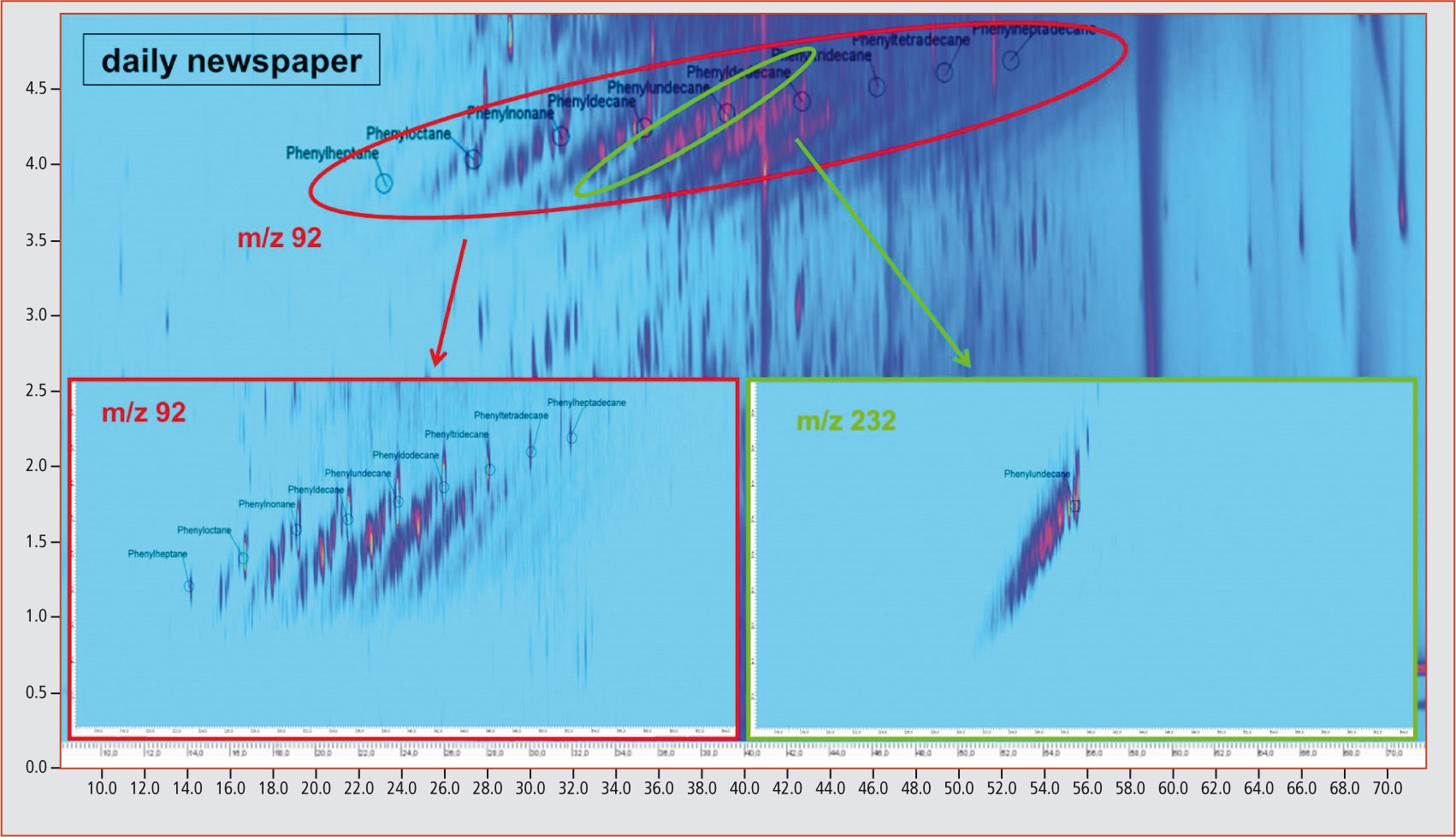 Figure 3: Phenylalkanes and differently substituted/branched analogs
Figure 3: Phenylalkanes and differently substituted/branched analogs
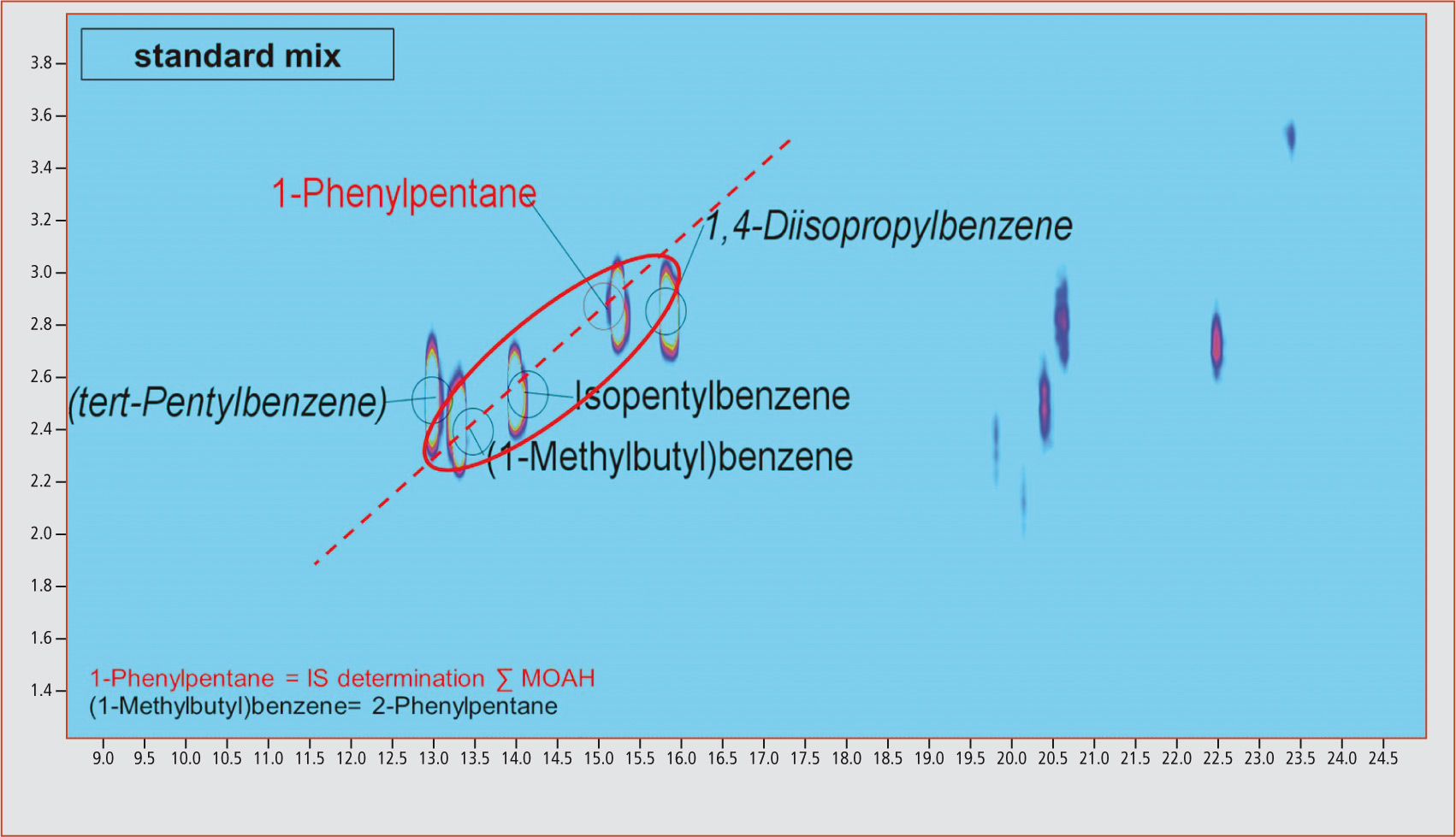 Figure 4: Detection of different branchings and substitutions exemplified by 1-phenylpentane
Figure 4: Detection of different branchings and substitutions exemplified by 1-phenylpentane
The results revealed that all samples were contaminated with mineral oil components, whereupon a comparison between fresh fiber and recycled samples showed that in recycled samples there was a considerably higher proportion of mineral oil compounds than in fresh fiber samples (figure 5).
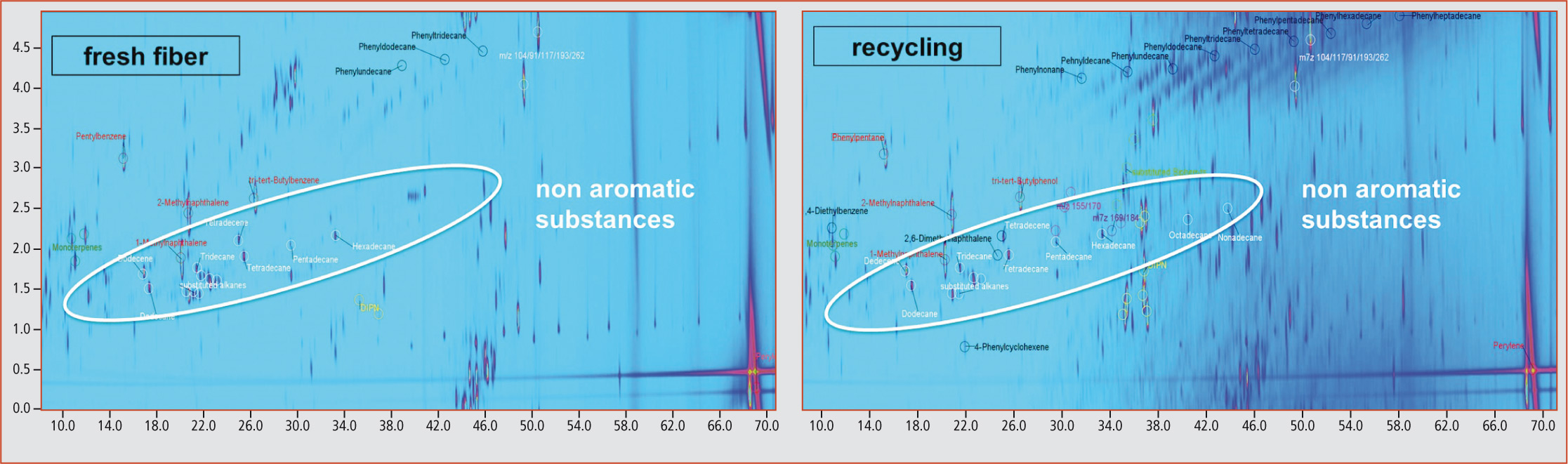 Figure 5: 2D-plot of a fresh fiber and a recycling sample
Figure 5: 2D-plot of a fresh fiber and a recycling sample
Phenylalkanes could be detected in all samples analyzed and, therefore, represent a substantial proportion of the MOAH fraction. The highest concentration for this compound class was detected in daily newspapers, followed by recycled papers. The concentration in fresh fiber samples was, as expected, considerably lower although still clearly detectable, which raised the question concerning the source of phenylalkanes in fresh fiber products.
Non-aromatic compounds also in the aromatic MOAH fraction
In addition to the phenylalkanes and aromatic substance groups such as biphenyls and diisopropylnaphthalenes, various non-aromatic compounds such as saturated hydrocarbons and terpenes were, however, also identified in the aromatic MOAH fraction (figure 5). In particular, saturated hydrocarbons that should have been constituents of the MOSH fraction could lead to false-positive values in the determination of the MOSH and MOAH sum parameters.
In addition to this problem, the complexity of the MOAH fraction becomes clearly apparent even in GCxGC separation, where chromatographically non-separated retention ranges occur. This is especially true for samples with high mineral oil concentrations such as recycling and newspaper samples.
Conclusion
In summary it can be said that multidimensional GCxGC is well able to obtain valuable information on the chemical and structural composition of mineral oil contaminations in paper and cardboard products. Nevertheless, more progress still needs to be made in the elucidation of the composition, which could eventually enable correct assessment of risk.
Authors
Dipl.-Ing. A. Jurek, Ao.Univ.-Prof. Dipl.-Ing. Dr. techn. E. Leitner, Institute of Analytical Chemistry and Food Chemistry, Technical University Graz, Austria
Literature
[1] R. Lorenzini, M. Biedermann, K. Grob, D. Garbini, M. Barbanera, I. Braschi, Food Addit Contam 30 (2013) 760-770
[2] S. Moret, M. Scolaro, L. Barp, G. Purcaro, M. Sander, L.S. Conte, Food Chem 157 (2014) 471-475
[3] M. Biedermann, K. Grob, Eur Food Res Technol 230 (2010) 785-796
[4] M. Biedermann, K. Grob, J Chromatogr A 1255 (2012) 76-99
[5] K. Fislier, F. Grundböck, K. Schön, O. Kappenstein, K. Pfaff, C. Hutzler, A. Luch, K. Grob, J Chromatogr A 1271 (2013) 192-200