Effect of mobile phase pH on reversed-phase HPLC separations of ionizable compounds
Changing the pH of a mobile phase is a powerful tool to obtain significant changes in the selectivity of a reversed-phase HPLC separation. Ionizable compounds, meaning acidic, basic or zwitterionic analytes will show extreme changes in retention depending on their pKa and pH of the mobile phase. Therefore, a wide pH range of the HPLC equipment offers additional possibilities for HPLC method optimization by exploiting selectivity changes at low, intermediate and high pH.
The mobile phase pH reflects the hydrogen ion [H+] concentration in solution. An acidic pH < 7 represents an increased [H+] concentration, while adding a base to pH > 7 lowers the [H+] concentration and suppresses ionization of basic analytes, as shown in the example of N-acetylprocainamide in figure 1 and methylparabene in figure 2.
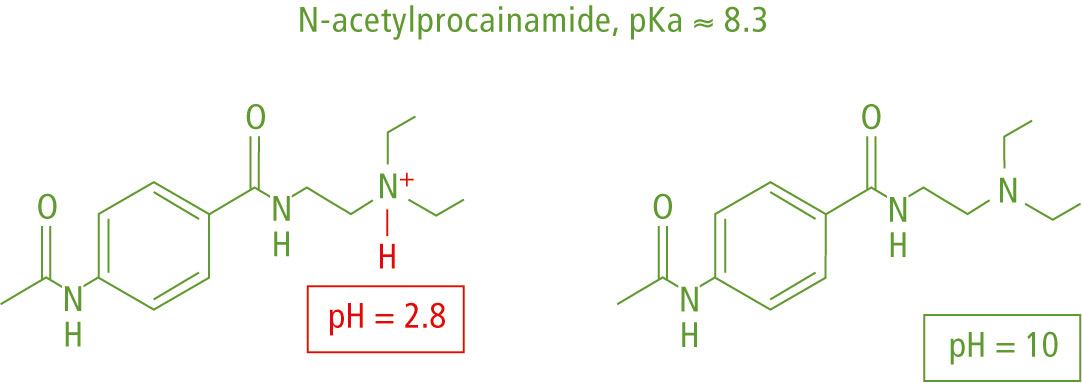 Figure 1: Ionization of N-acetylprocainamide in acidic or basic pH
Figure 1: Ionization of N-acetylprocainamide in acidic or basic pH
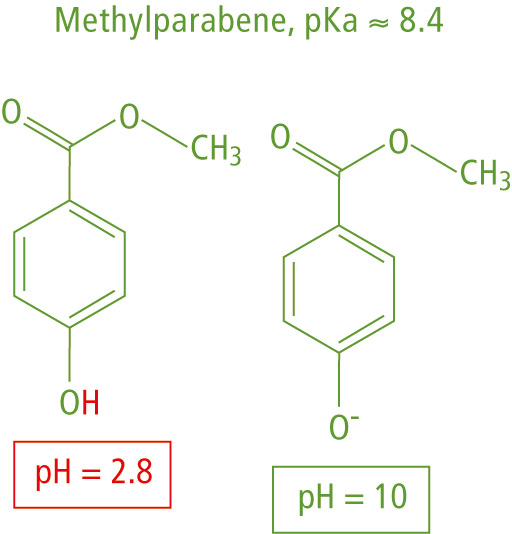 Figure 2: Ionization of methylparabene in acidic or basic pH
Figure 2: Ionization of methylparabene in acidic or basic pH
As the extent of analyte ionization changes, so does the retention behavior in reversed-phase HPLC separations. Since the ionized form is more polar, it is retained less on alkylated, non-polar stationary phases. Ion suppression by decreasing the pH for acids, or increasing it for basic analytes will significantly lengthen retention as demonstrated in the application example shown in figure 3.
A mixture of seven pharmaceuticals was separated in acidic or basic mobile phase conditions. While the neutral analytes showed no marked change in retention time, the basic analytes, namely nizatidine, N-acetylprocainamide and reserpine were retained much longer in basic conditions. Methylparabene moved forward once the phenol group was ionized (figures 2 and 3).
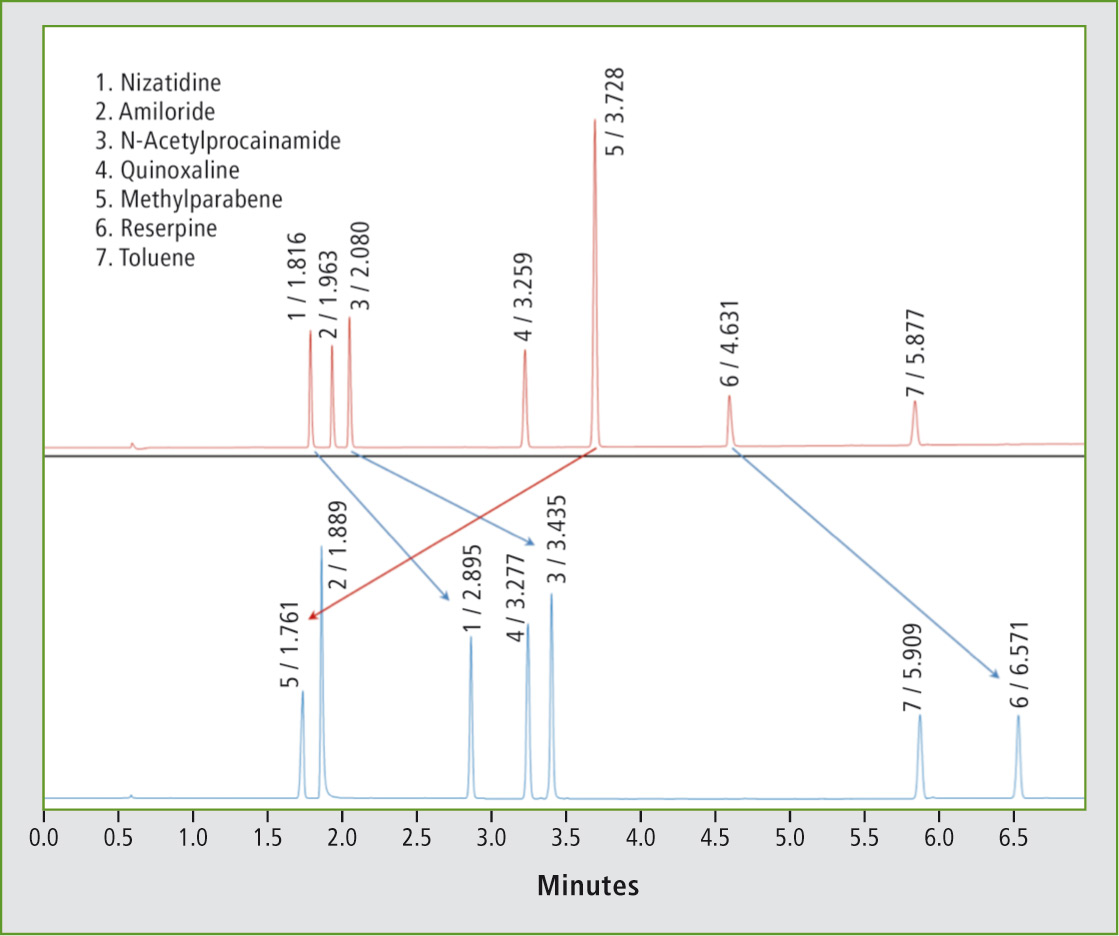 Figure 3: Separation of seven pharmaceuticals in a) acidic or b) basic mobile phase conditions
Figure 3: Separation of seven pharmaceuticals in a) acidic or b) basic mobile phase conditions
Ion suppression can also be used to considerably improve the peak shape of ionizable compounds, as charged analytes undergo secondary interactions with the stationary phase, which can lead to strong peak tailing. Neutral or uncharged compounds will normally elute as sharp, symmetrical peaks. In any case, care should be taken to adjust the mobile phase pH in a range that is about 2 pH units away from the analyte pKa, where a compound is 50 % ionized. This can give rise to split peaks, due to different retention behavior of the ionized and non-ionized form.
Instrumentation: Nexera X2 series UHPLC system, wide pH version
Column: ACE Excel 3 Super C18, 100 x 2.1 mm
Mobile phase: a) acidic: A: 10 mM HCOONH4, pH 2.8 in H2O and B: in MeCN/H2O (90:10 v/v)
b) basic: A: 0.1 % NH3, pH ~ 10 in H2O and B: in MeCN/H2O (90:10 v/v)
Gradient: 3 – 100 % B in 7 min
Cycle time: 11 min
Flow rate: 0.42 mL/min
Temperature: 40 °C
Injection volume: 2 µL
Sample: ~ 0,3 mg/mL of each compound in MeCN/H2O (5:95)