Extending GCMS to the analysis of Active Pharmaceutical Ingredients
Analysis of N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA) in pharmaceutical substances by HS-GCMS
 Headspace-Autosampler HS-20
Headspace-Autosampler HS-20
N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA) are the simplest dialkylnitrosamines. They continue to be released as a by-product and contaminant from various industries and from municipal wastewater treatment plants. Major releases of NDMA and NDEA are caused by the manufacture of pesticides, rubber tires, alkyl amines and dyes. These compounds are also produced as a by-product in the manufacture of Active Pharmaceutical Ingredients (API’s). These compounds are classified as a Group 2A carcinogen (probable human carcinogen) by the World Health Organization.
Recently, some drug products were discovered to have been contaminated with NDMA and NDEA (figure 1). It is believed that they were introduced into the finished products during the manufacturing process. The contamination exceeded by far the regulatory exposure limits specific to the drug products. Consequently, medical agencies across Europe as well as the US Food and Drug Administration (USFDA) withdrew all affected drug products from the market.
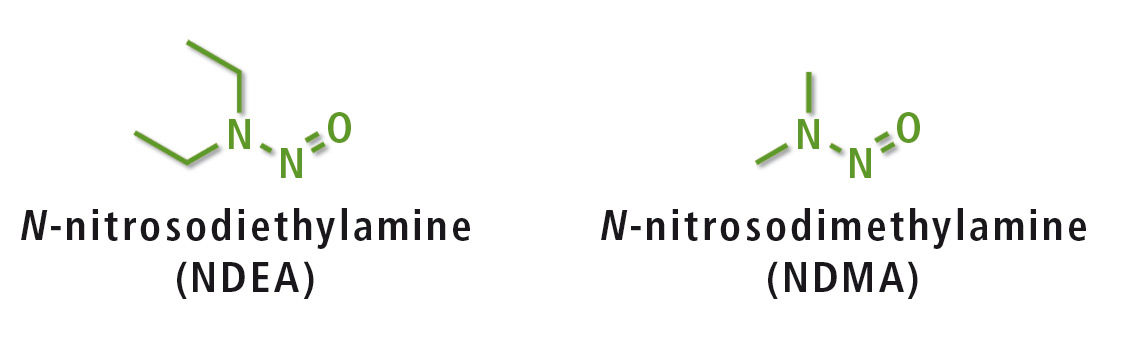 Figure 1: Structure of NDEA and NDMA
Figure 1: Structure of NDEA and NDMA
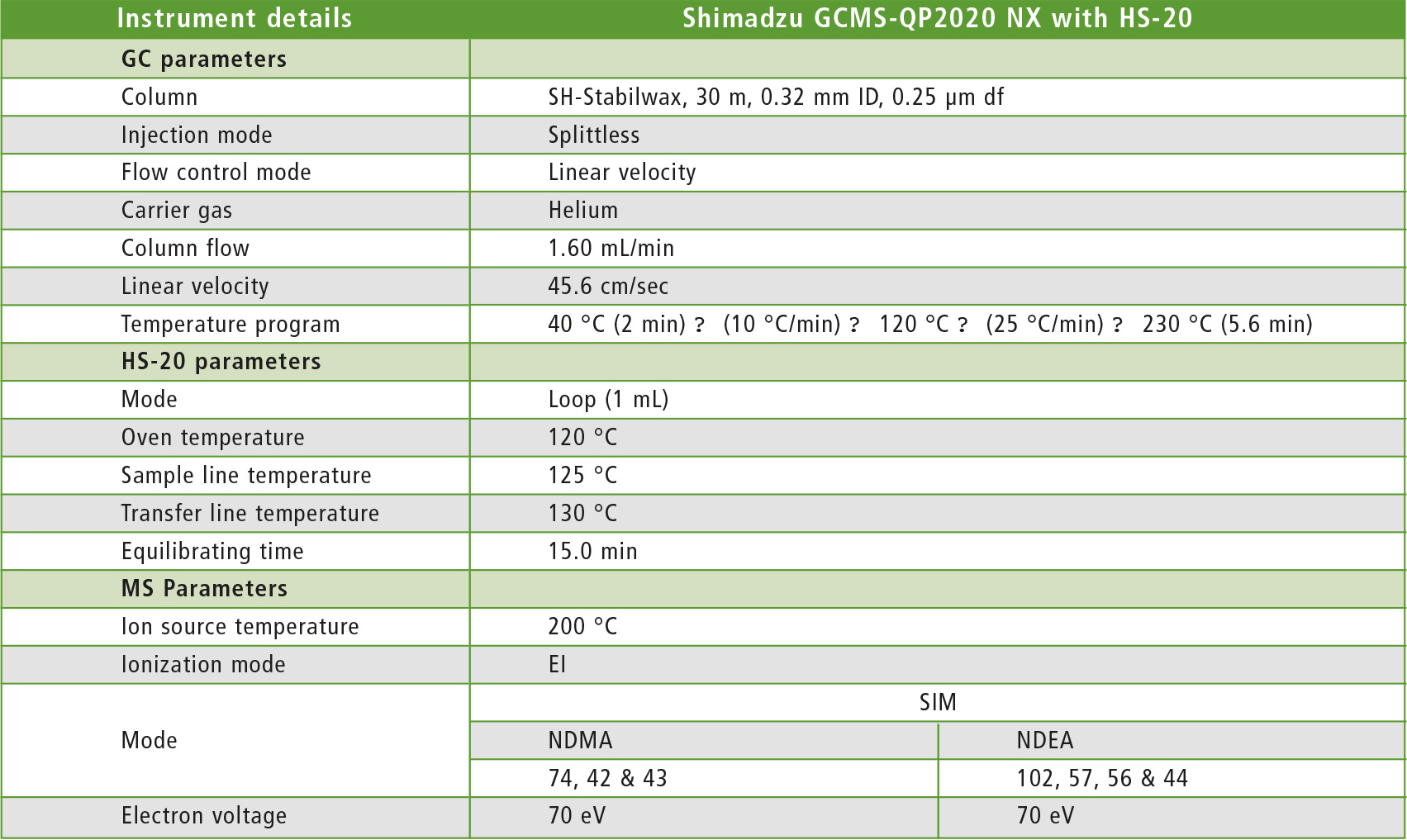 Table 1: GCMS-QP2020 NX operating conditions
Table 1: GCMS-QP2020 NX operating conditions
It is therefore essential to have a sensitive, accurate, reliable and robust analytical method available based on a suitable technique. A Headspace Gas Chromatography Mass Spectrometry (HS-GCMS) method has been developed to detect the presence of NDMA in drug substances. In this experiment, the pharmaceutical API’s (Valsartan, Losartan and Olmesartan-Medoxomil) subject to contamination with NDMA and NDEA are analyzed.
Experimental
This study was conducted using a Shimadzu GCMS-QP2020 NX single quadrupole with HS-20. A commercial mixture of NDMA and NDEA was used to prepare calibration curves ranging from 2.5 – 10 µg/L. The standards were prepared in N,N-Dimethyl sulfoxide. Instrument operating conditions are shown in table 1.
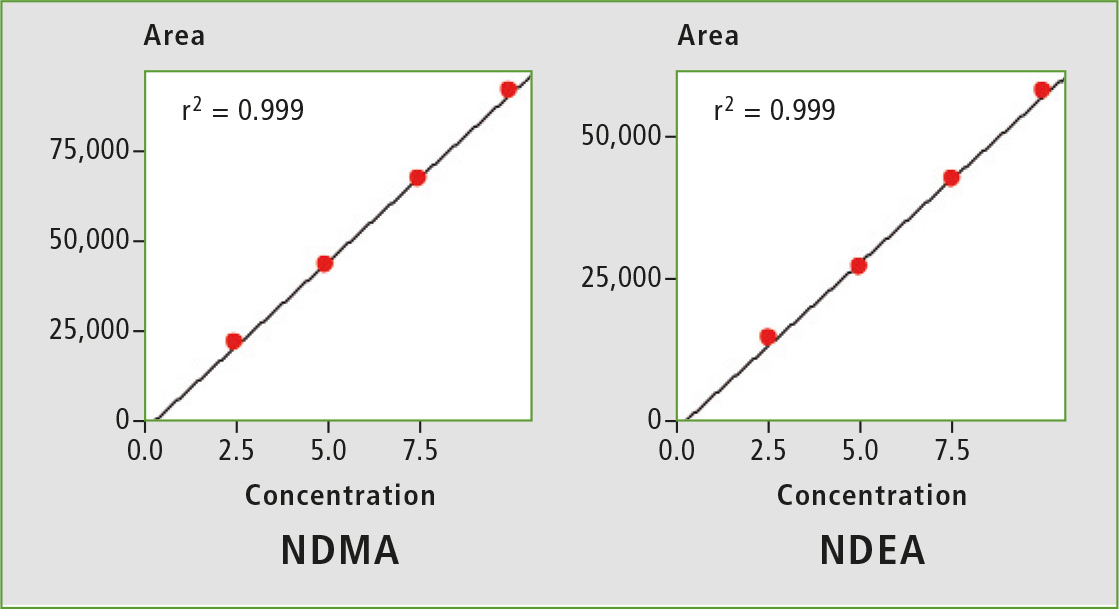 Figure 2: Calibration curves from 2.5 ppb to 10 ppb
Figure 2: Calibration curves from 2.5 ppb to 10 ppb
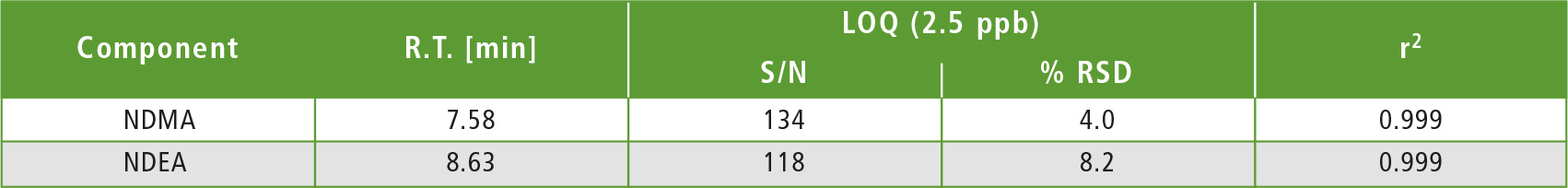 Table 2: LOQ summary
Table 2: LOQ summary
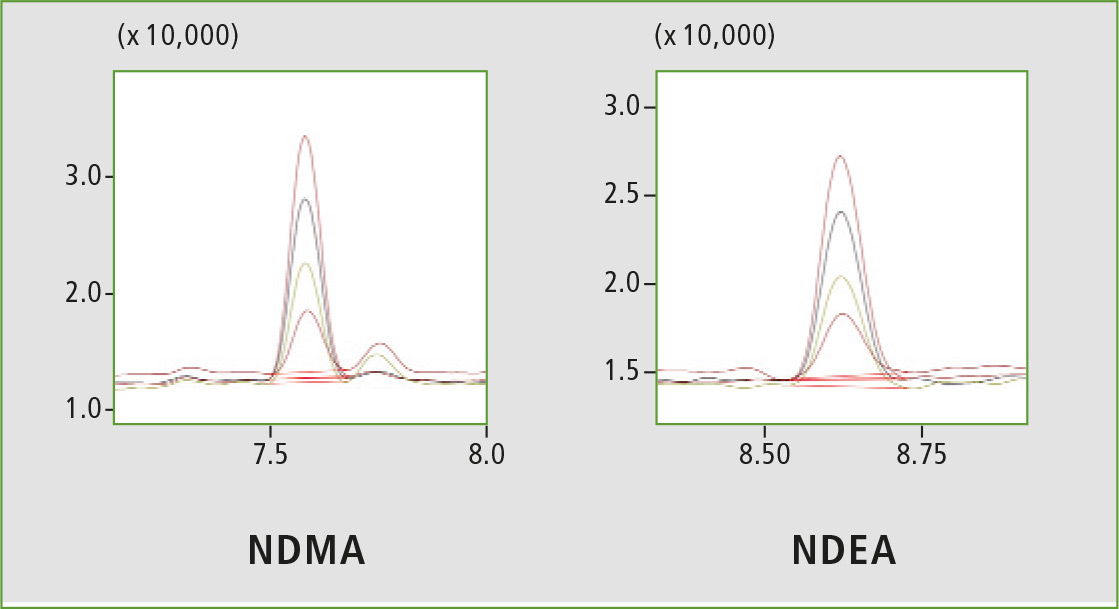 Figure 3: Chromatographic overlay from 2.5 ppb to 10 ppb
Figure 3: Chromatographic overlay from 2.5 ppb to 10 ppb
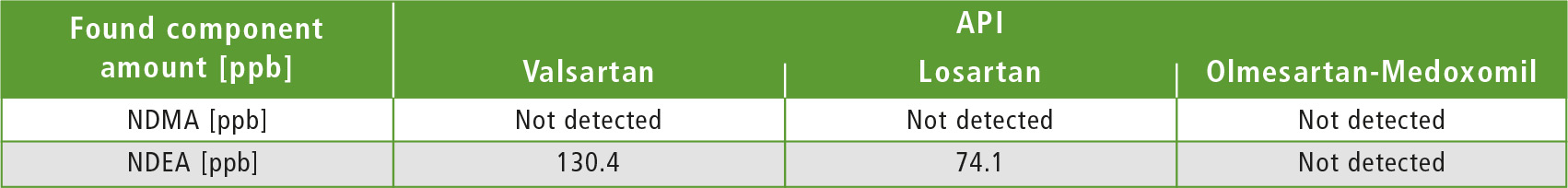 Table 3: Results obtained by sample analysis
Table 3: Results obtained by sample analysis
Linearity of the calibration curve
A four-point calibration curve of NDMA and NDEA from 2.5 to 10 µg/L was analyzed using the conditions described in table 1. Retention times, correlation coefficient and LOQ (limit of quantification) established from S/N (signal/noise) and % RSD (relative standard deviation) are shown in table 2. The calibration curves established for both components with r2 greater than 0.999 for calibration levels 2.5, 5.0, 7.5 and 10.0 ppb are shown in figure 2. Figures 3 and 4 depict chromatographic overlay of all linearity levels and six replicates of 5.0 ppb solution respectively.
Sample analysis and recovery test
Three API samples (Valsartan, Losartan & Olmesartan-Medoxomil) were weighed individually in a 20 mL headspace vial, to which 2 mL of DMSO were added as diluent to make 5 % w/v (weight/volume) solution. For the recovery tests, individual API’s were weighed in 20 mL headspace vials which were spiked with 2 mL 2.5 ppb, 5.0 ppb and 10.0 ppb NDMA and NDEA standard solutions respectively, and were then subjected to HS-GCMS analysis. Tables 3 and 4 show results of the sample analysis and accuracy study for 3 API’s.
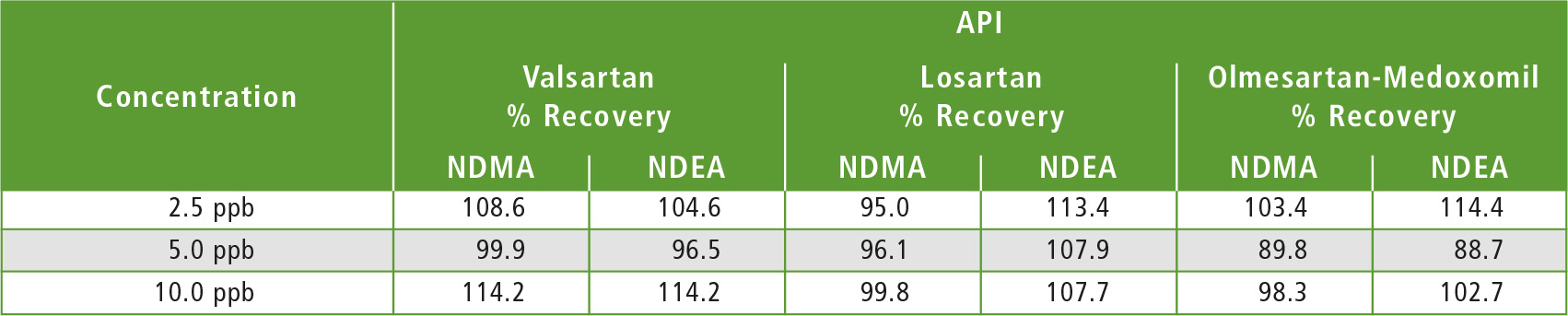 Table 4: Results obtained in the accuracy study for three API’s
Table 4: Results obtained in the accuracy study for three API’s
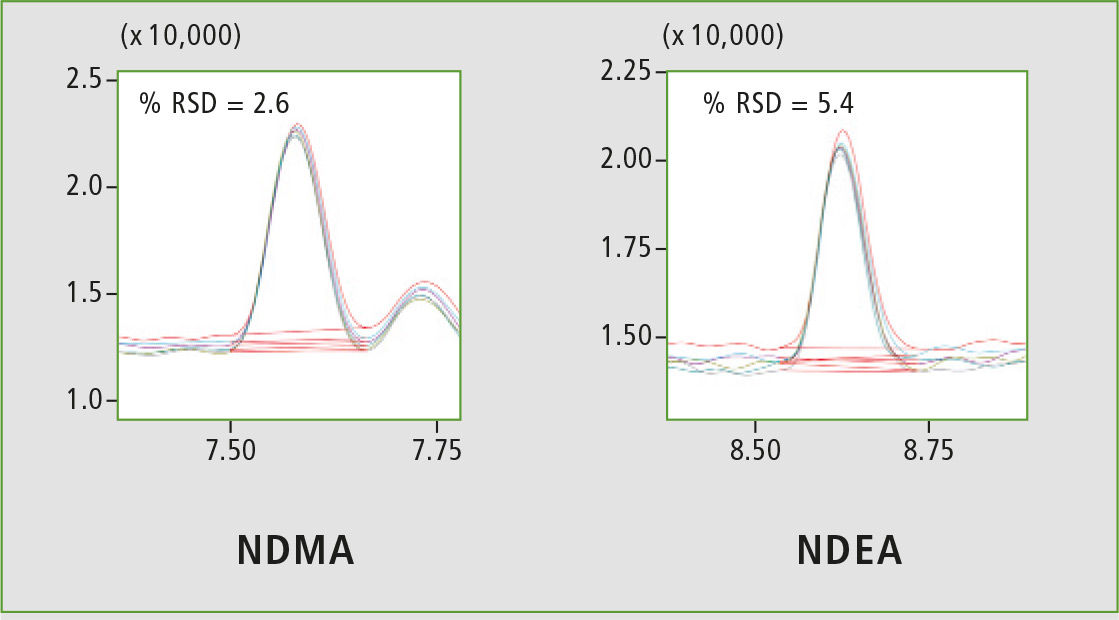 Figure 4: Chromatographic overlay of six replicates of 5 ppb
Figure 4: Chromatographic overlay of six replicates of 5 ppb
Conclusion
- A highly sensitive method was developed for quantitation of genotoxic impurities namely NDMA and NDEA in pharmaceutical API’s using Shimadzu’s GCMS QP2020 NX with HS-20 headspace autosampler.
- The SIM method developed can be used for screening of NDMA and NDEA in various pharmaceutical products.
- Ultra-Fast scanning and ASSP™ (Advanced Scan Speed Protocol) features of Shimadzu GCMS enabled a sensitive, selective, fast, reproducible and linear method of analysis.