New tool for healthcare and cosmetic products
SPFCalculator software for Shimadzu UV-VIS instrumentation

A new UV-VIS instrumentation software for the healthcare and cosmetic segments outperforms the new regulations to be applied from July 2013. These adjustments will be fixed by the European Community, relating to the production and marketing of cosmetics [1]. Other regulations are the Colipa [2], FDA [3], Boots Star Rating [4] and JCIA (Joint Commission International Accreditation – Health Organisation). All of them are part of the SPFCalculator Software which even helps to calculate some physical properties. This product has been designed in cooperation with the Aqualis software house in Milano, Italy.
The SPFCalculator presents 17 parameters requested for the qualification of a sun protection product. The program includes more information on the sun screen product than is required by the guidelines. The calculator is easy to handle and just needs a simple training for users. The software can be updated based on changes in the regulations. The seventeen parameters which can be calculated are shown in table 1.
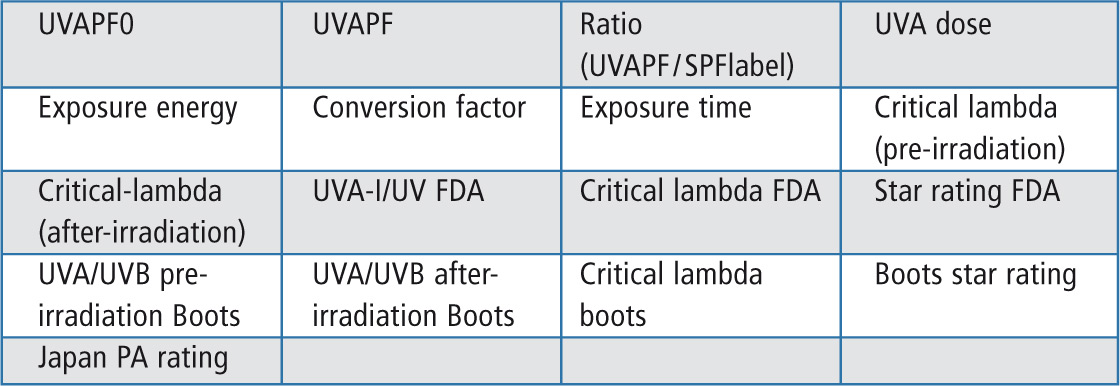 Table 1: Parameter selection for the SPF report
Table 1: Parameter selection for the SPF report
Users can easily test the sun protection product and change its formulation where necessary, before carrying out the very expensive in vivo measurements.
Shimadzu can provide a complete easy to handle solution: a package containing the UV-2600 instrument, the ISR-2600 integrating sphere in combination with UVProbe and the SPFCalculator. It is a total solution in accordance with European Cosmetics Associations requirements. The strength of the package is that other parameters such as color, appearance, quality of packaging etc. can be determined using the same combination.
References
[1] Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products
[2] Colipa, (European Cosmetics Trade Association) ‘In Vitro Sun Protection Methods’ group
[3] Sunburn Protection Factor (SPF). Food and Drug Administration (United States). 2009-04-30; ‘Questions and Answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the U.S.’, updated 6/23/2011
[4] Measurement of UVA: UVB Ratio According to the Boots Star Rating System (2008 Revision), Boots, Nottingham, UK, 2008