How to keep a clear view
Quality assurance applying spectroscopy for Eye-Cleaning solutions

The eyes are one of the most important sensory organs. But they are very sensitive to accidents, necessitating precautions in case of emergency. An example of an eye accident could be ingress of particles or chemicals (particularly in laboratories). In this case, the eyes need to be rinsed with a special Eye-Cleaning solution.
The quality assurance of such a solution is very important to ensure that the rinsing helps rather than causing even more problems, e.g. through introduction of microorganisms which can cause serious infection of the eyes. To prevent the solution from containing microorganisms, the filling has to be aseptic. It is an efficient way to fill a plastic bottle directly after extruding and moulding it into the common bottle shape. Filling the bottle at this time with the temperature-stable solution results in fast cooling of the bottle material. The productivity of this manufacturing process known as blow fill seal (bfs) is much higher than when working with air as cooling medium or flushing the bottle with rinse solution, quite apart from the possibility of higher contamination risks. These are some of the reasons that Holopack® (near Stuttgart, Germany) offers this technique (The ready bottled sample is shown in figure 1).
 Figure 1: Analyzed product: Sterile Eye-Cleaning solution bottled by bfs-technique
Figure 1: Analyzed product: Sterile Eye-Cleaning solution bottled by bfs-technique
A different aspect in quality assurance is the screening of the contents. Normally, the amounts of salts included in an Eye-Cleaning solution are well known by the manufacturers. But in order to exclude weighing errors or to control the stability and real concentration of the elements in the final solution, an additional control step is indispensable for increased safety. Therefore, it is necessary to determine the element concentrations of sodium (Na), potassium (K), magnesium (Mg) and calcium (Ca) in a fast and reliable way.
AAS or ICP-OES?
For the analysis of Na, K, Mg and Ca it is desirable to use both techniques, Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) and Atomic Absorption Spectroscopy (AAS). With the ICPE-9000 system and the AA-7000 dual atomizer instrument, Shimadzu meets the demands of quality assurance aspects.
The AA-7000 is the system of choice for economic analysis of up to 4-5 elements. When six or more elements should be determined quickly, the ICPE-9000 system is more applicable (compare figure 2). Additional criterions for decisions can be present, e.g. the different detection limits of the analytical systems. But in case of the Eye-Cleaning solution this is not a point because all four analytes are high concentrated to imitate the human tear fluid to avoid eye-irritations.
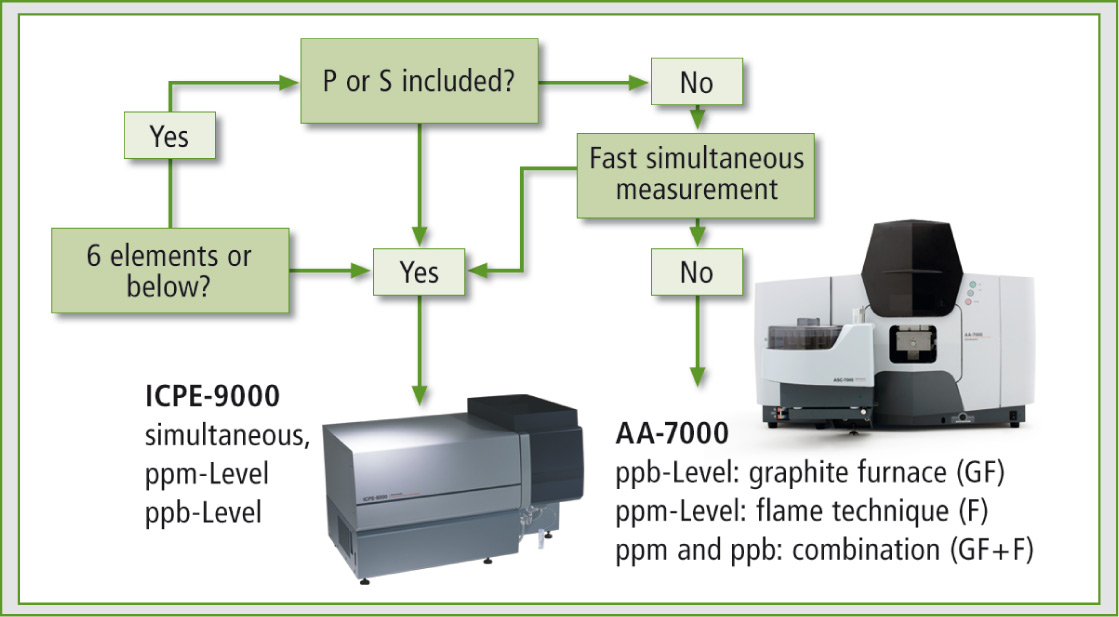 Figure 2: The flow chart indicates the suitable element analysing technique
Figure 2: The flow chart indicates the suitable element analysing technique
Analytical Task
The analytical challenge of this sample is the highly varying levels of the elements Na, K, Mg and Ca. For example, sodium is present in a high ppm range whereas the concentration of magnesium is 100 times lower (Diagram 1). Based on the fact that sample preparation nowadays has to be highly automated with the lowest amount of work, one dilution only of the sample should be sufficient to determine all elements, instead of several dilutions to match each analytical working range.
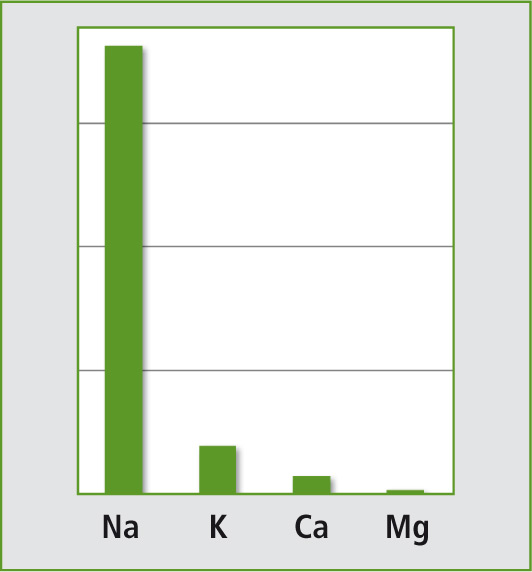 Diagram 1: Element ratios in Eye-Cleaning solutions
Diagram 1: Element ratios in Eye-Cleaning solutions
A 100-times dilution was selected for working with the Eye-Cleaning solution. In this way, it is possible to determine magnesium (now 0.36 ppm), calcium and potassium with flame atomic absorption technique using the AA-7000F. For determination of sodium, the observed absorption wavelength has to be changed to the less-sensitive 330.3 nm-line, which is of factor 200 less sensitive in comparison to the most common 589.0 nm-line.
Micro Sampling
To have more scope in method development and individualization in the final steps of the method optimization process, Shimadzu’s Micro Sampling is perfectly suited to measure the Eye-Cleaning solution. For example, when applying ionisation buffers as prescribed for determination of sodium in drinking water (in accordance with German Drinking Water Directive).
The AA-7000F in combination with the ASC-7000 sample preparation station allows the automated flame Micro Sampling method (Figure 3). In this method, flame atomic absorption analysis is conducted with small sample volumes (50-90 µL), whereas in the conventional flame method (hereafter “flame continuous method”), the sample is continuously aspirated with a flowrate of approximately 8 mL/min, and larger sample volumes are needed for aspiration.
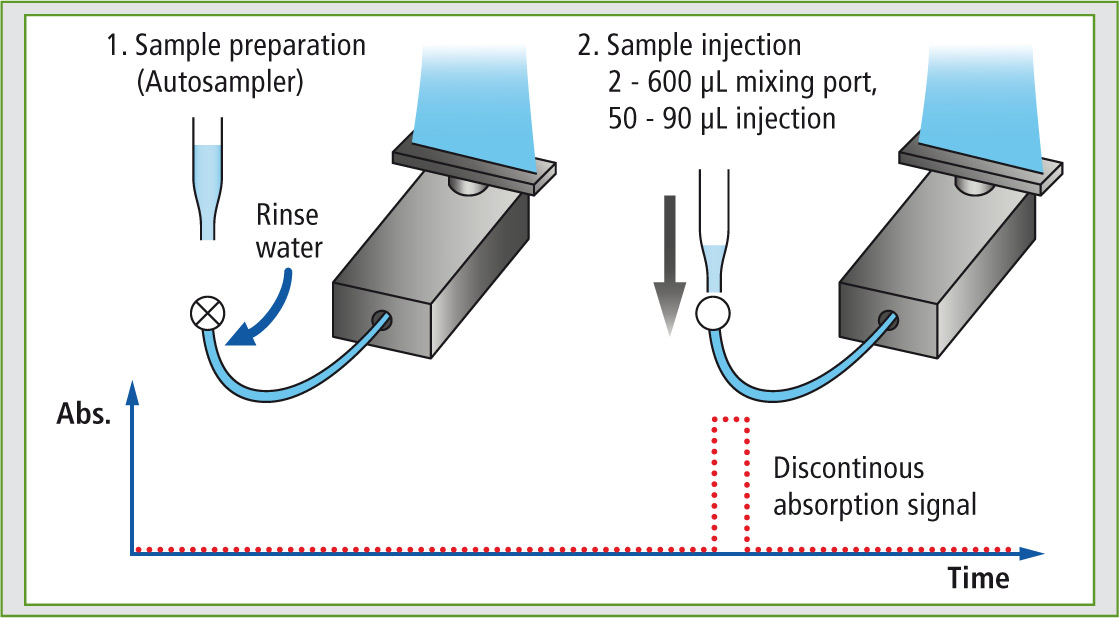 Figure 3: Flame Micro Sampling method
Figure 3: Flame Micro Sampling method
In the first step (Figure 3) the sample can be diluted and water, ionization buffers and/or standard solutions (standard addition method) can be added. The total mixing volume is 600 µL. The resulting solution is injected in step two.
The flame Micro Sampling method has several advantages compared to the flame continuous method. Analysis is possible with a small amount of sample, and when the autosampler is used, automatic further dilution of the sample and automatic addition of buffer solutions are possible in order to compensate for interferences. Moreover, since only a small amount of sample is introduced, the flame Micro Sampling method is effective for analysis of high matrix samples which may cause clogging of the burner in the flame continuous method, such as the Eye-Cleaning solution.
Final method and results
Method parameters providing the best measured results are summarized in table 1. Using these parameters, it was possible to measure over hours with no clogging of the burner head, as can happen when measuring high matrix samples with common continuous flame method.
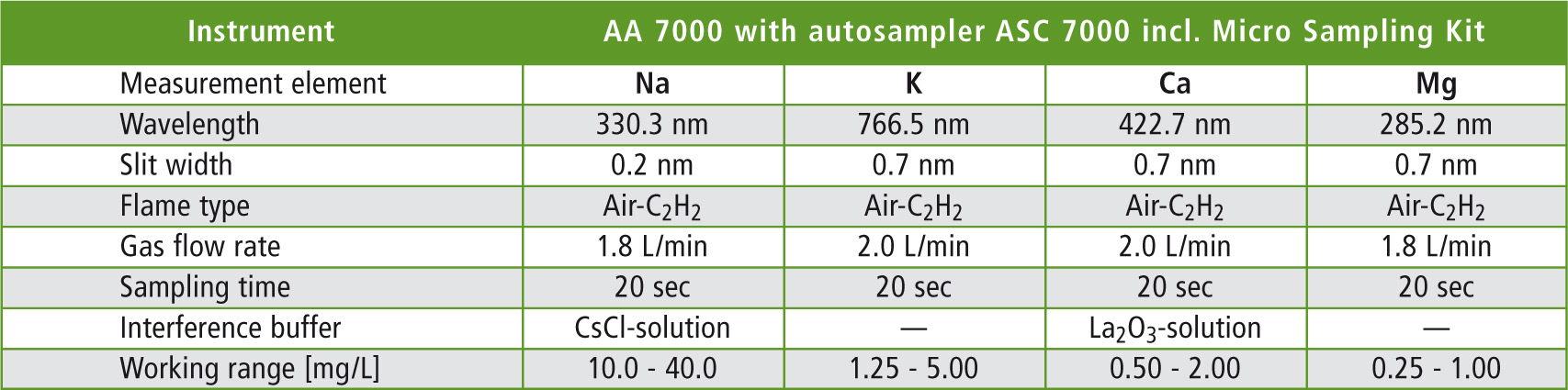 Table 1: Final method parameters for quality assurance of highly concentrated elements in Eye-Cleaning solutions
Table 1: Final method parameters for quality assurance of highly concentrated elements in Eye-Cleaning solutions
To illustrate measurement results, the calibration plus the absorption signal of magnesium are shown in Figure 4. The recovery was in a good range for all elements included in this research (Table 2). The AA-7000 in combination with Micro Sampling is a “state of the art” atomic absorption spectrophotometer for high precision measurements of element concentrations in Eye-Cleaning solutions as well as in mineral waters (see Shimadzu News 01/2011). In general, this method is an interesting tool for clinical or pharmaceutical applications as it complies with FDA CFR 21 Part 11, and the possibility to optimize all parameters including individual sample dilution even in a multi-elemental measurement sequence emphasises the Micro Sampling technique.
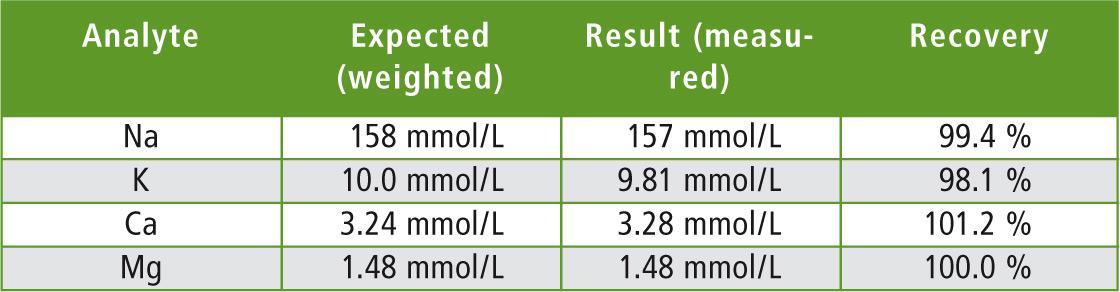 Table 2: Measurement results for the Eye-Cleaning solution
Table 2: Measurement results for the Eye-Cleaning solution
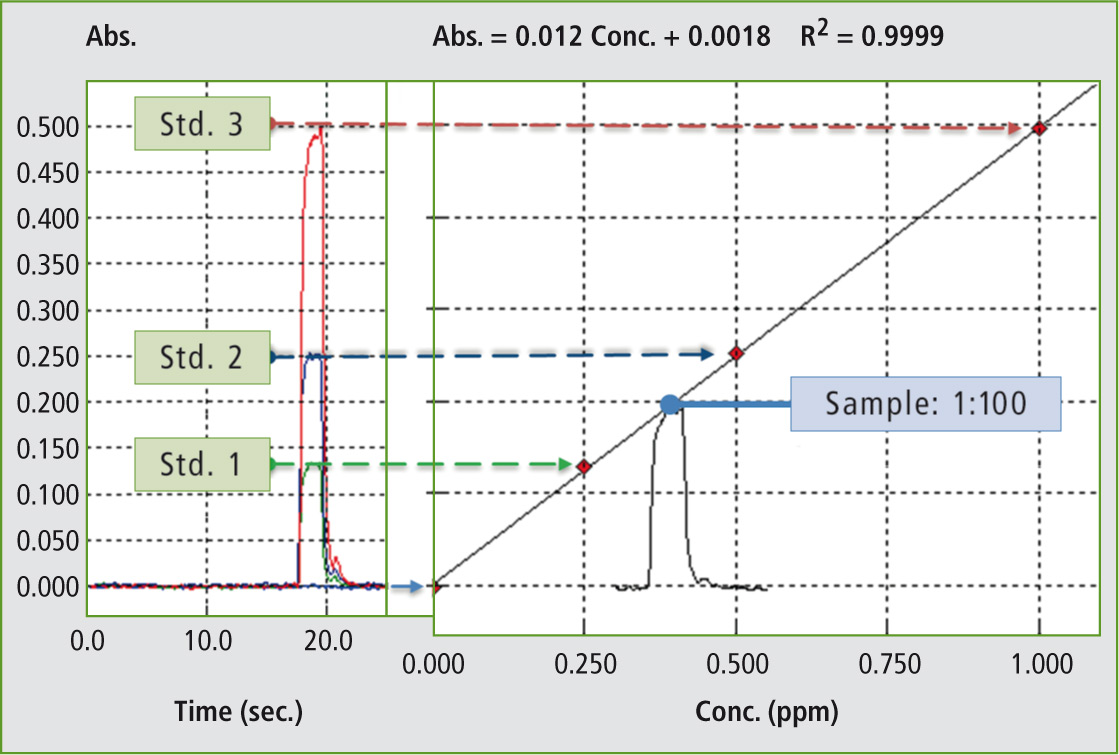 Figure 4: Absorption signals and calibration pattern for magnesium
Figure 4: Absorption signals and calibration pattern for magnesium