How cleaning validation prevents contamination
Cleanliness is an important issue in the production of any type of drug, since highest purity and careful handling of substances and active substances are essential in the pharmaceutical industry. The basic request is the effective removal of residual products incurred during production. A clean plant reduces contamination of the medicine. When using a batch process, subsequent cleaning is particularly important due to the production of several drugs in the same plant. Contamination by the previous product must be prevented. Cleaning validation ensures the efficiency of the cleaning process.
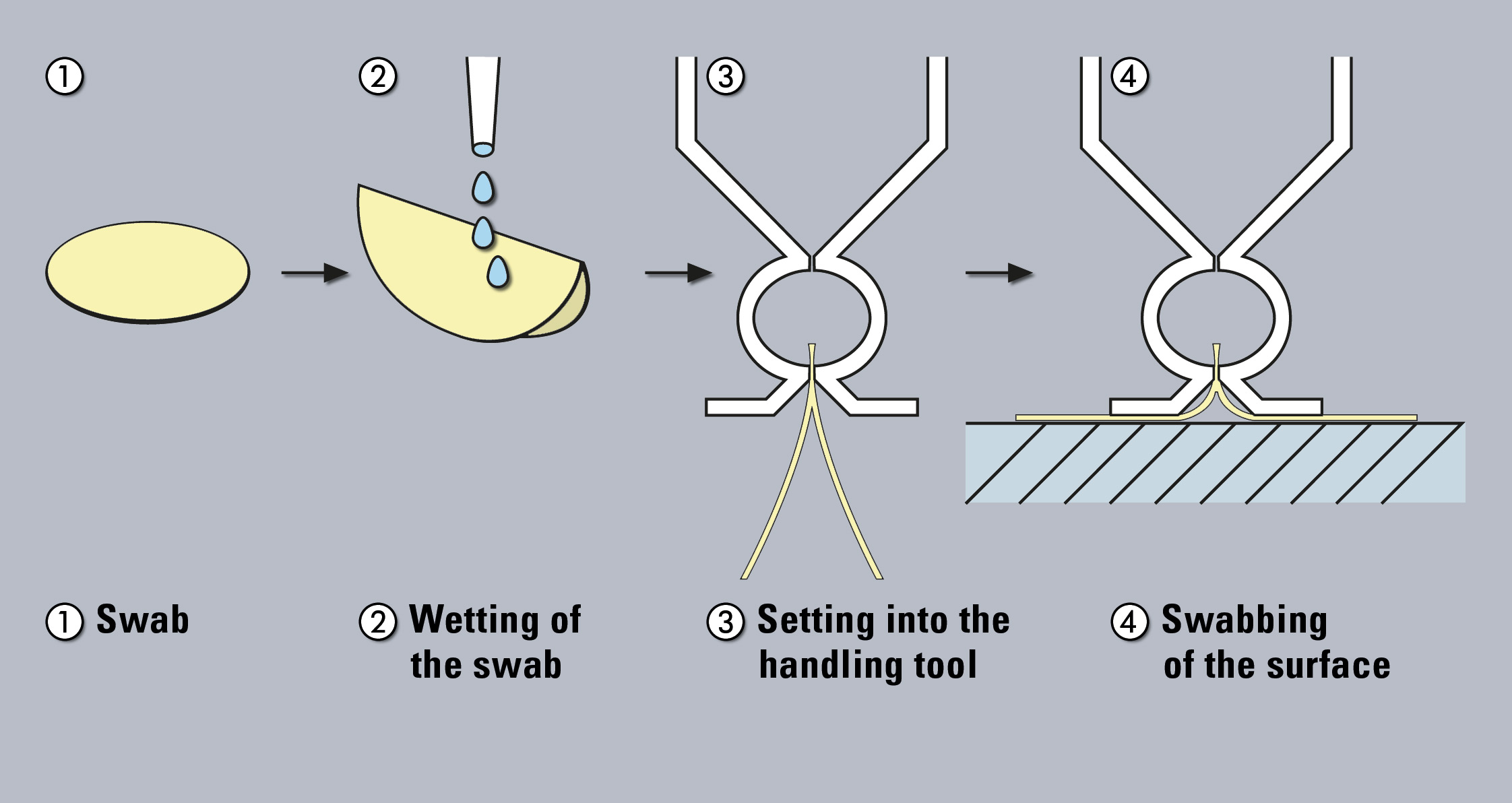 Figure 1: Sampling with swabs
Figure 1: Sampling with swabs
Cleaning validation is not a new issue. In 1963, the Good Manufacturing Practice (GMP) already declared:
“Equipment shall be maintained in a clean and orderly manner” (GMP Reg../FDA Part 133.4). A similar section on equipment cleaning was included in the 1978 CGMP regulations.
The implication of cleaning validation has lately been increasingly significant since the pharmaceutical industry develops more and more active substances which are effective even in low concentrations. Proof of contamination needs to be validated by analytic methods which are sensitive enough to define accepted limits of residues. A typical criterion is 10 mg/L or 1/1,000 of therapeutical doses of the medication.
Based on the large variety of pharmaceutical drugs, different analytical methods are applied in the analysis of residues. In particular, HPLC and TOC analysis have been proven to demonstrate the effectiveness of the rinsing process.
Cleaning process
Selection of the right cleaning process is the decisive factor in effectively cleaning the plant. Two processes are normally used.
1. CIP (Clean In Place)
The cleaning is done automatically without dismounting the plant. In this case, the plant has to have a CIP-design (rinsing system with recycling possibility and no dead volumes) which usually requires a high investment.
On the other hand, optimizing of time, temperature, cleaning agent and solvents is possible, resulting in very effective cleaning. The automatic rinsing enables a standardized and validated procedure.
2. COP (Cleaning Out of Place)
With COP, the complete plant needs to be dismantled and all components cleaned individually. This procedure requires more time and staff resources. Due to the individual cleaning, standardization and validation is more difficult but low investment and the opportunity to inspect all components visually are among the benefits.
Sampling and analytics
Depending on the cleaning process, several facilities can be used for sampling to check the cleaning efficiency. The easiest and fastest way is the analysis of the final rinse solution. Depending on the solvent used, subsequent analysis can be carried out with HPLC or TOC.
The swab method is applied to test the surface of the production container. In this way, areas can be evaluated, which are difficult to clean but reasonably accessible and the level of contamination of residues per given surface can be determined. In addition, residues that are “dried out” or insoluble can be sampled by physical removal. Traditionally, the swabs are subsequently extracted and the resulting solution is further analyzed. However, use of a TOC-solid module with glass-fibre swabs free of any carbon enables direct analysis of the swabs.
TOC-Analysis
Total Organic Carbon measures the carbon content of organic matter present in different matrices. The carbon content of the sample is oxidized to carbon dioxide and detected with a NDIR-detector. Water samples can be measured easily and very quickly. (Typical measurement time is four minutes).
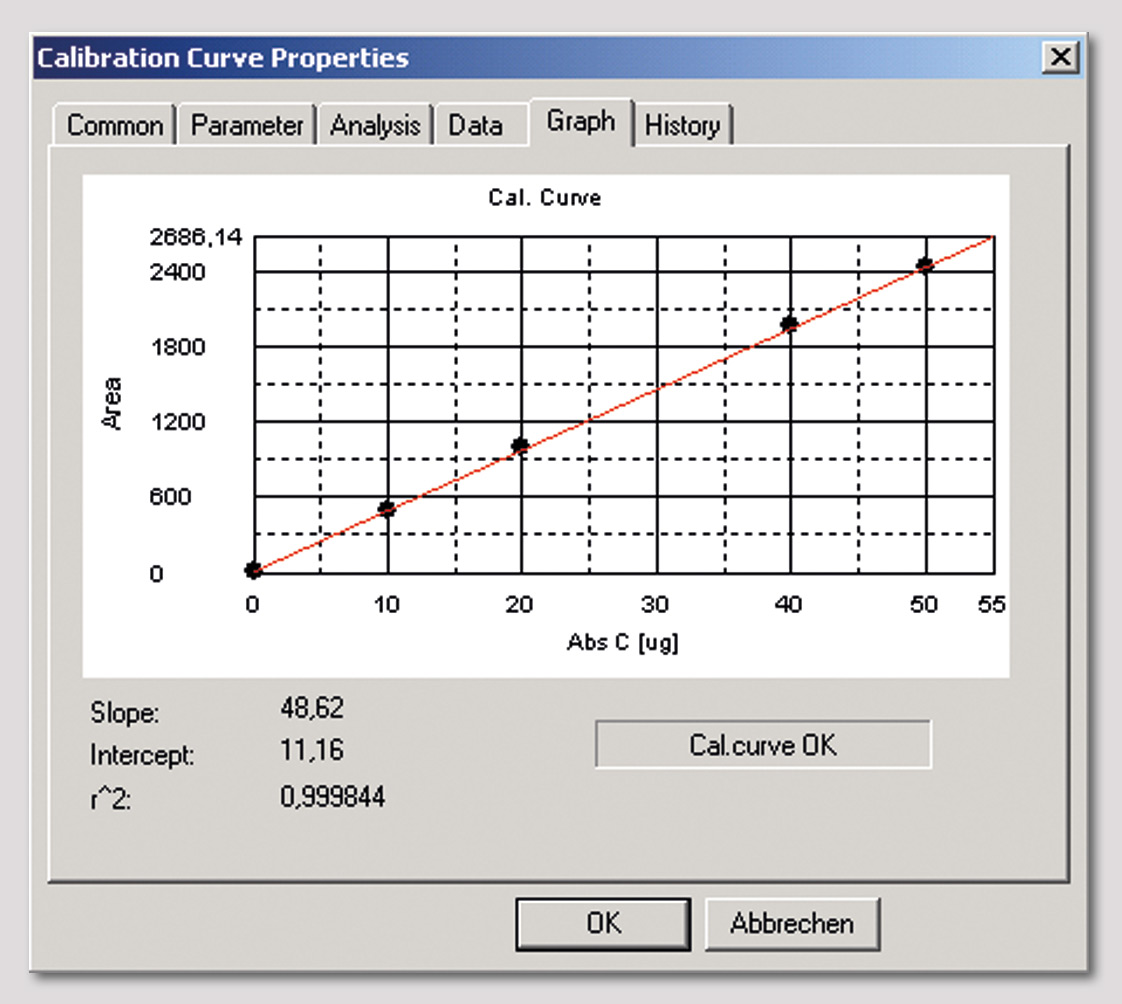 Figure 2: Calibration curve of TOC-solid module up to 50 µg
Figure 2: Calibration curve of TOC-solid module up to 50 µg
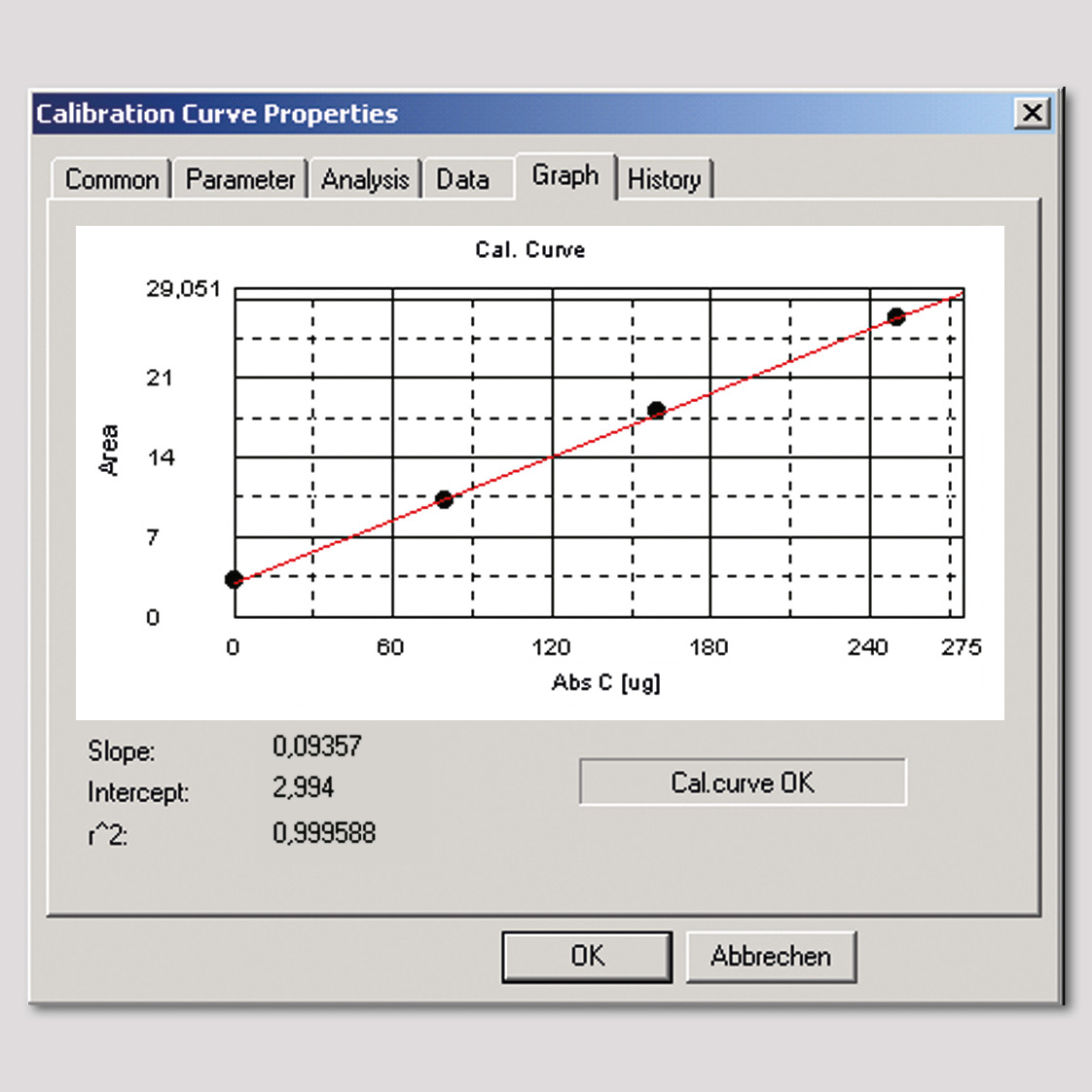 Figure 3: Calibration curve of TOC-solid module up to 250 µg/L
Figure 3: Calibration curve of TOC-solid module up to 250 µg/L
In cleaning validation, TOC-values cover contamination of the pre-product and the remaining rinsing solution. If this result is below the defined limit, further analysis is unnecessary. However, if the result exceeds the limit, HPLC-analysis is needed to identify the source of contamination.
Shimadzu’s modular TOC-L series simplifies TOC-analysis, regardless of how samples of final rinse or swab method are measured. The TOC-L is based on the proven technologies of catalytic combustion (680 °C) and NDIR-detection. In this way, it is possible to detect low molecular mass acids and invisible particles. Due to its measuring range of up to 30,000 mg/L (detection limit: 4 µg/L), the instrument is not only suitable for the low range but also for analysis of highly contaminated samples, for example during the cleaning validation process.
TNM (Total Nitrogen Measurement) is an analytical technique used for the determination of total water-borne nitrogen (TN) by means of oxidative combustion chemiluminescence. Both combustion tube and oxidation catalyst are shared with TOC (Total Organic Carbon) analysis. The well-known TOC applications used for Cleaning Validation purposes are therefore complemented by the determination of nitrogen in the related samples. This enhances the analytical information obtained from the cleaning validation samples, in particular proteins and amino acids since nitrogen is an essential part of these compounds.
Since the number of insoluble compounds in the pharmaceutical industry is increasing, direct determination of swabs with the TOC-solid module has been gaining more significance. In this case, a carbon-free glass-fibre swab is wetted with purified water and the defined surface is swiped according to a prescribed procedure. The swab is then folded and placed in a clean ceramic boat and inserted in the TOC-solid module. The system configuration and calibration curve are selected depending on the expected concentration or the defined limiting value. The calculated carbon content now corresponds directly to the area of the swabbed surface.
Conclusion
Cleaning validation is an essential part of good manufacturing practice, avoiding cross contamination in the pharmaceutical production process. Fundamental components of cleaning validation are the analytical methods applied. Regardless of the type of cleaning procedure, Shimadzu’s instrumentation and software supports the examination of cleaning validation samples with TOC. Specific software tools help to perform the partly complex analysis and support documentation and archiving of the analysis results.