Determination of sodium, potassium and calcium
Analysis of Na, K and Ca with flame atomic absorption spectrometry in microsampling mode
Determination of elements in pharmaceutical products such as crystalloid solutions typically applies atomic absorption spectrophotometers such as AA-7000. Concentrations of sodium (Na), potassium (K) and calcium (Ca) are the same as in the body fluids/extracellular fluids and need to be monitored carefully.
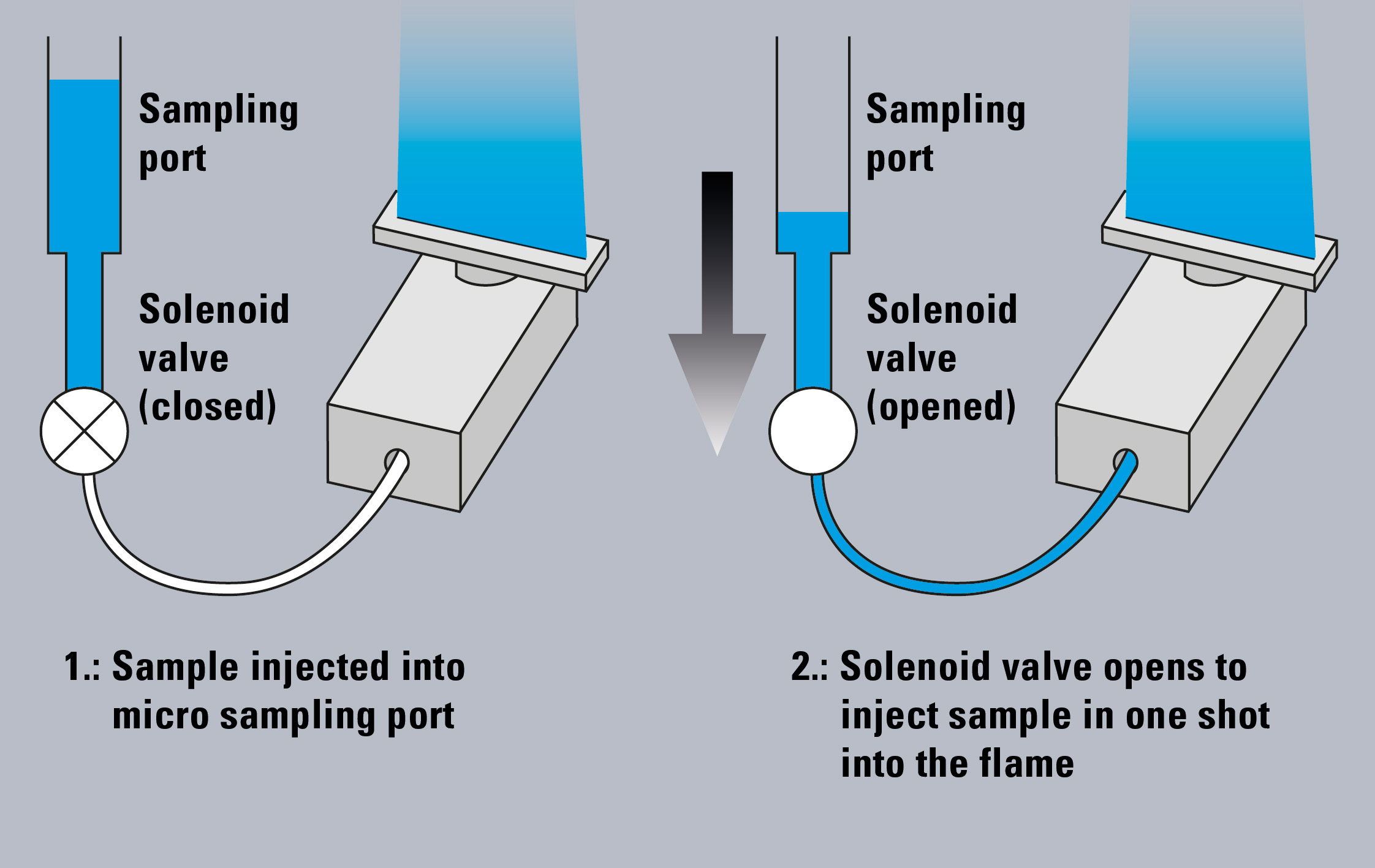 Figure 1: Microsampling method on AA-7000F
Figure 1: Microsampling method on AA-7000F
Crystalloid solutions are rapidly excreted by the kidneys and appear virtually unchanged. Furthermore, the solutions are suitable for use in dissolution and parenteral administration of drugs. Upon administration the crystalloid solutions are rapidly eliminated. They have therefore proven to be the solutions of choice for administration of drugs. Table 1 shows typical element concentrations of crystalloid solutions.
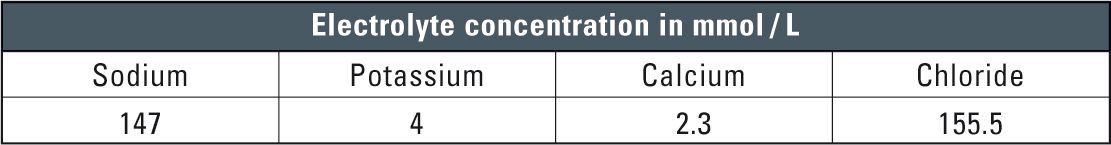 Table 1: Typical example of a full electrolyte solution/crystalloid solution
Table 1: Typical example of a full electrolyte solution/crystalloid solution
The AA-7000 combined with the ASC-7000 sample preparation station enables the automated flame micro sampling method (Figure 1). In this method, flame atomic absorption analysis is conducted with small sample volumes (2 – 90 µL), while in the conventional flame method (hereafter “flame continuous method”), the sample is aspirated continuously with a flow rate of approximately 8 mL/min and larger sample volumes are needed for aspiration.
The right choice: the flame micro sampling method
When compared with the flame continuous method, flame micro sampling has several advantages: the analysis is possible with a small amount of sample, and when the autosampler is used, automatic dilution of the sample and automatic addition of buffer solutions are possible in order to compensate for interferences. Moreover, since only a small amount of sample is introduced, the flame micro sampling method is effective for analysis of high matrix samples which may cause clogging of the burner in the flame continuous method.
The method is the right choice for determination of alkaline and alkaline earth elements in crystalloid solutions.
Sodium, potassium, and calcium belong to the essential mineral substances in the human organism. These elements are influential in the generation of enzymes and hormones, control osmotic pressure in tissues and body fluids and are important for the exchange procedures in the cell membranes.[1]
Particularly during surgery, severe loss of extracellular fluids (interstitial fluid and blood) is critical and must be compensated by crystalloid solutions having a similar composition to the extracellular fluids. Blood losses may also be compensated by these solutions provided the quantity of the loss is not too critical.
Reliable determination of elements in high matrix samples
Control of Na, K, and Ca in crystalloid solutions according to the European Pharmacopoeia has been performed with the Shimadzu atomic absorption spectrophotometer AA-7000 in a fully automatic multi element sequence. The blank, standards and the test samples have been analyzed in the direct calibration method.
All solutions were placed in the autosampler ASC-7000 and mixed automatically with the corresponding reagents necessary to achieve reliable results. In the case of sodium and potassium 40 µL of CsCl-solution (12.65 g CsCl + 50 mL HCl (d = 1.16) filled up to 500 mL volume with H2O) was added for a 400 µL mixing volume of standard and sample solution, homogenized before injection to the flame. In the case of calcium, a La2O3-solution (5.875 g La2O3 + 50 mL HCl (d = 1.12) filled up to 250 mL volume with H2O ) was used.
Instrumental parameters and measuring conditions were set element-specific from the system software. These conditions are automatically set for each element including optimized burner height and gas flow rates and are listed in table 2.
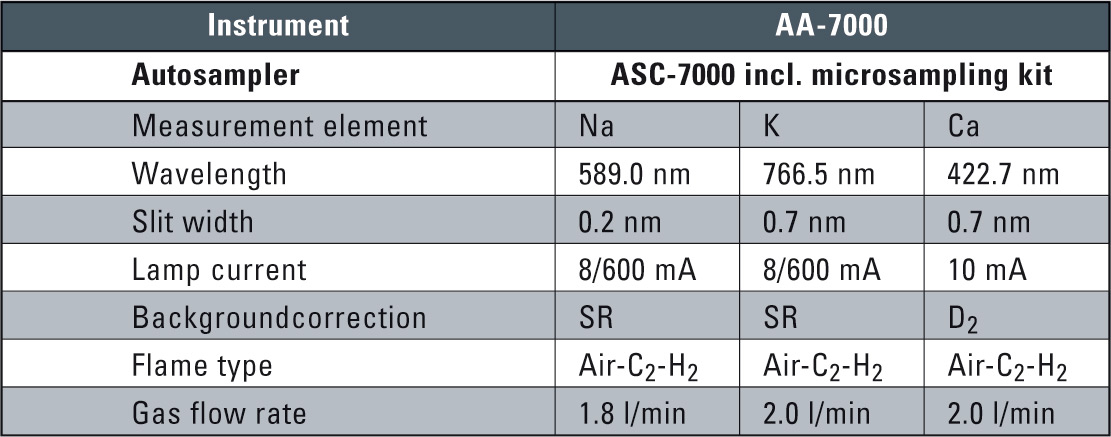 Table 2: Instrument and Analytical Conditions
Table 2: Instrument and Analytical Conditions
Under these conditions a series of crystalloid solutions has been analyzed to demonstrate that the AA-7000 is an effective tool for the reliable determination of elements in high matrix samples during routine analysis.
[1] Mineralstoffe und Spurenelemente, Leitfaden für die ärztliche Praxis, Bertelsmann, 1992