Method transfer and validation according to Quality by Design requirements
DryLab®2010 and Nexera – a powerful combination
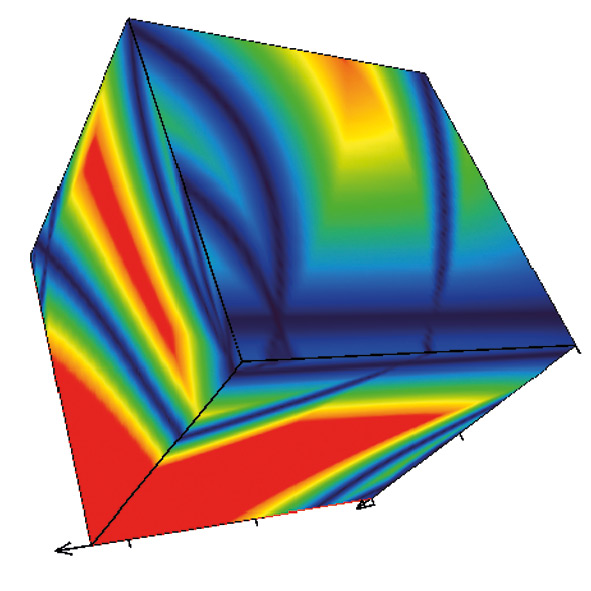 Fig. 1: Cube generate by DryLab2010 – the read area describes the robust area of the method
Fig. 1: Cube generate by DryLab2010 – the read area describes the robust area of the method
In 2004, the FDA issued the PAT initiative (process analytical technology), intending to change development processes also in the pharmaceutical industry. Nowadays, the Quality by Design concept as a logical continuation is discussed and influences current and future actions. Guidelines issued by ICH (International Conference on Harmonization) and other regulatory authorities request the application of QbD principles.
The complexity of parameters in a HPLC method makes it very difficult to change and modify some of the parameters once this method is validated. Adapting a separation from conventional to ultra-high pressure LC and the related effect are difficult to describe and predict. DryLab2010 is a valuable tool to simplify this process, based on designed experiments to predict method changing effect and describe the ‘Design Space.’
Shimadzu and the Molnar Institute in Berlin cooperated in applying well-known DryLab2010 software with the new generation of UHPLC instruments.
 Figure 2: Shimadzu Nexera with LCMS 2020
Figure 2: Shimadzu Nexera with LCMS 2020
With Drylab2010 it is now possible to simultaneously optimize several measured experimental parameters (tG, T, pH or ternary composition of eluent B) with six other derived factors (column length, -ID, dp, flowrate, dwell volume, extra column volume) to further define the Design Space of a separation. DryLab2010 uses two-dimensional tG-T resolution maps [2]. By applying three of them, three-dimensional resolution spaces can be created in which the combined influence of three parameters can be assessed and optimized.
DryLab applies QbD principle since 20 years with a systematic evaluation of parameters influencing method performance (selectivity, resolution).
DryLab simplifies and speeds up the process of developing excellent chromatographic separations by enabling users to model changes in separation conditions using their personal computer. The time-consuming laboratory runs that are typically required to achieve a satisfactory separation or development of a complete method are replaced with instantly generated chromatograms corresponding to conditions that can be selected individually.
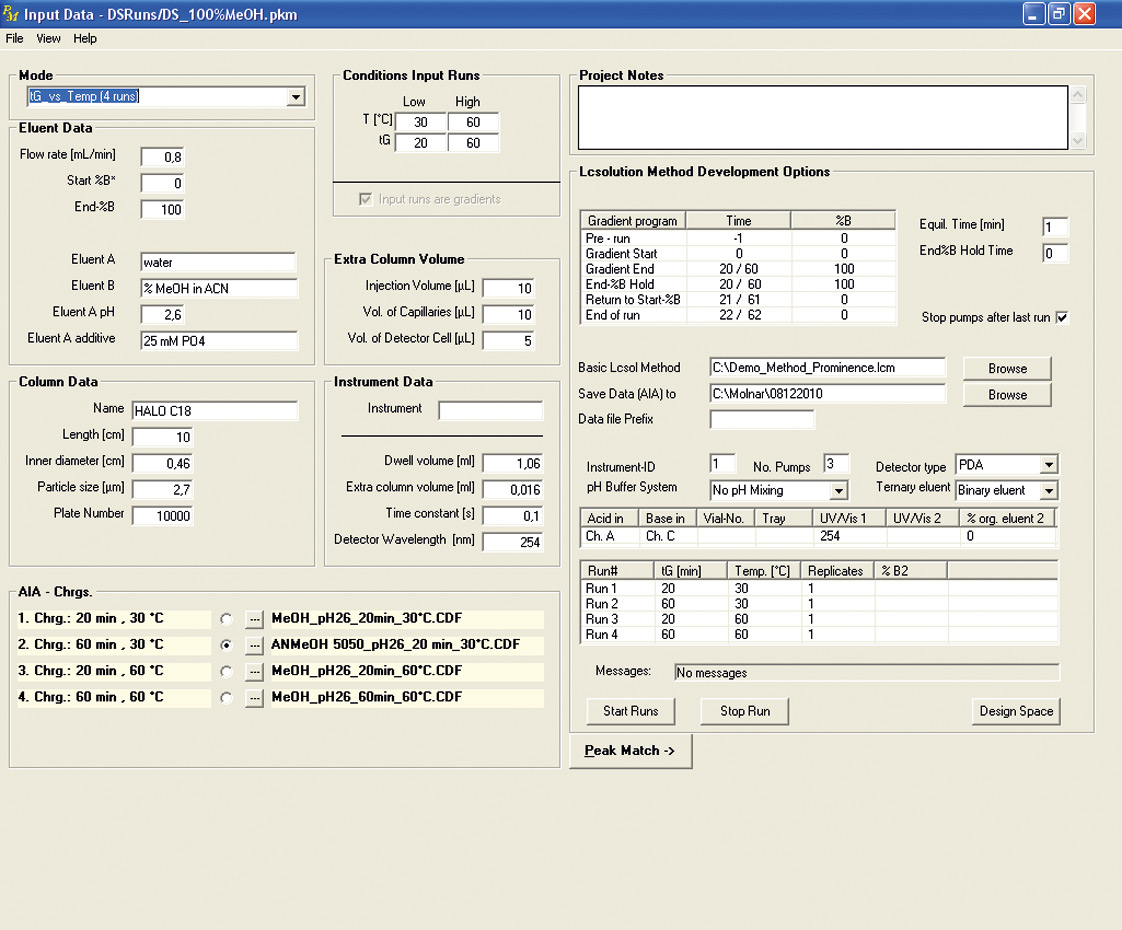 Figure 3: Interface of Peak Match with Shimadzu’s LabSolution software parameters
Figure 3: Interface of Peak Match with Shimadzu’s LabSolution software parameters
DryLab is useful in almost all chromatography applications in the lab. The software helps to
- quickly find a good set of separation conditions
- develop complete method in minimal time
- evaluate method robustness
- shorten run times
- transfer methods to better and more modern instruments such as Shimadzu Nexera
- easily carry out validation of your methods
- find the best separation conditions for any component in a mixture
- fine tune or troubleshoot existing methods
- make the method fit to its purpose
- adjust methods for ageing columns.
The Cube
The cube represents over a million simulated chromatograms and evaluates three parameters simultaneously. It finds the best separation with a mouse click and helps to find the optimum solvent composition (e.g. Ratio between Acetonitril / Methanol). The necessary input data for these 3D models is depicted in figure 4: three tG-T models at three different values of a third parameter are needed.
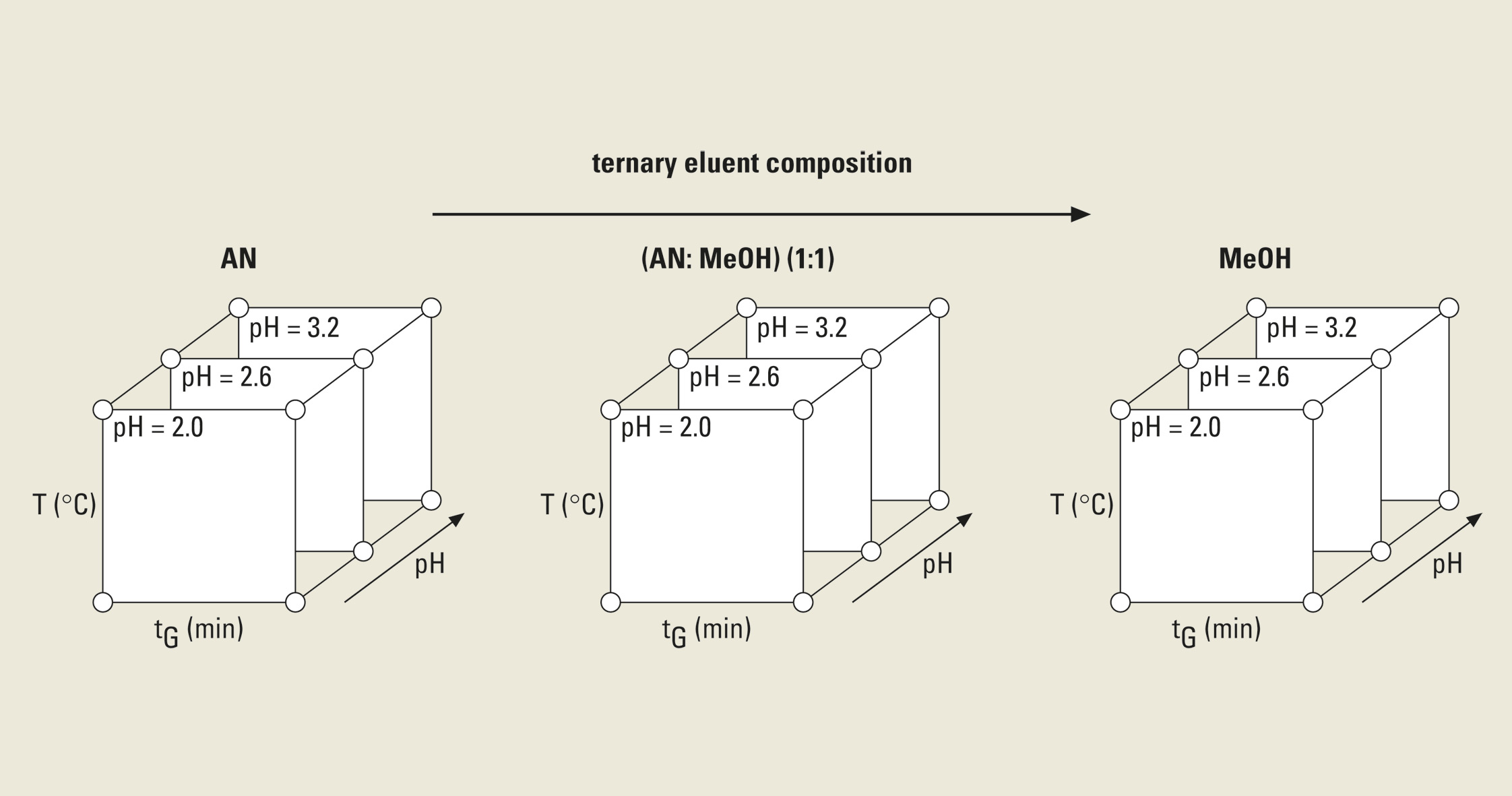 Figure 4: Experimental design for a three-dimensional HPLC method optimization
Figure 4: Experimental design for a three-dimensional HPLC method optimization
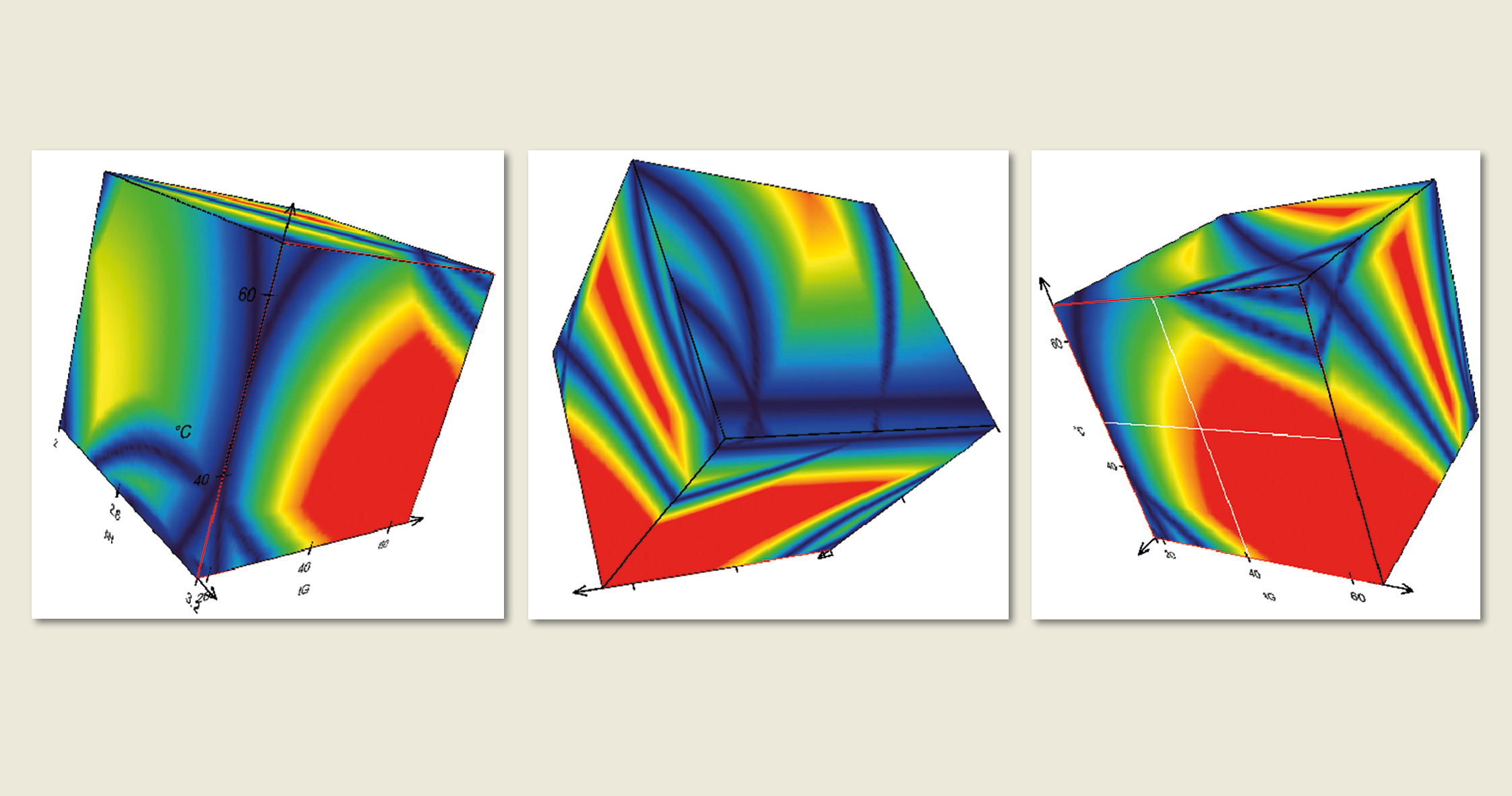 Figure 5: Design Space Visualization with DryLab®2010. Each point in these 3D resolution spaces corresponds to a highly accurate chromatogram
Figure 5: Design Space Visualization with DryLab®2010. Each point in these 3D resolution spaces corresponds to a highly accurate chromatogram
Design of Experiments
Preliminary evaluation determined that the most influential experimental variables on critical resolution (separation between critical peak pair) were
- the gradient time (tG)
- the temperature (T)
- the pH value
- the ternary eluent composition.
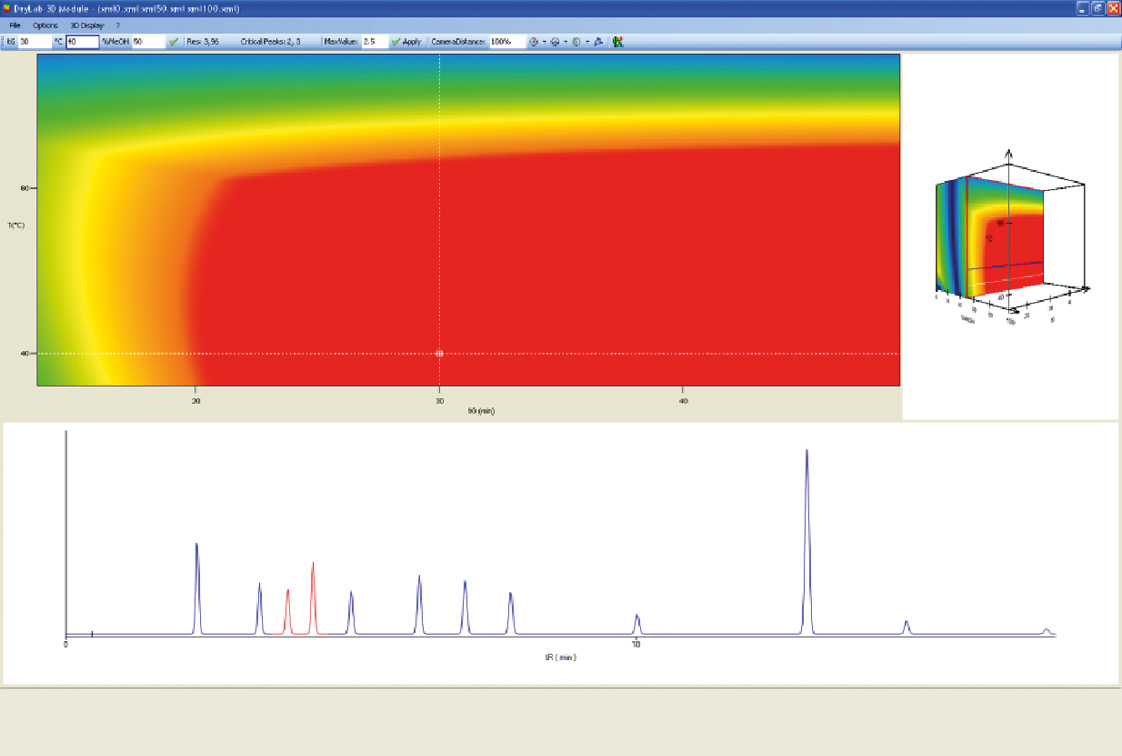 Figure 6: DryLab 3D model. The best chromatogram by one mouse click.
Figure 6: DryLab 3D model. The best chromatogram by one mouse click.
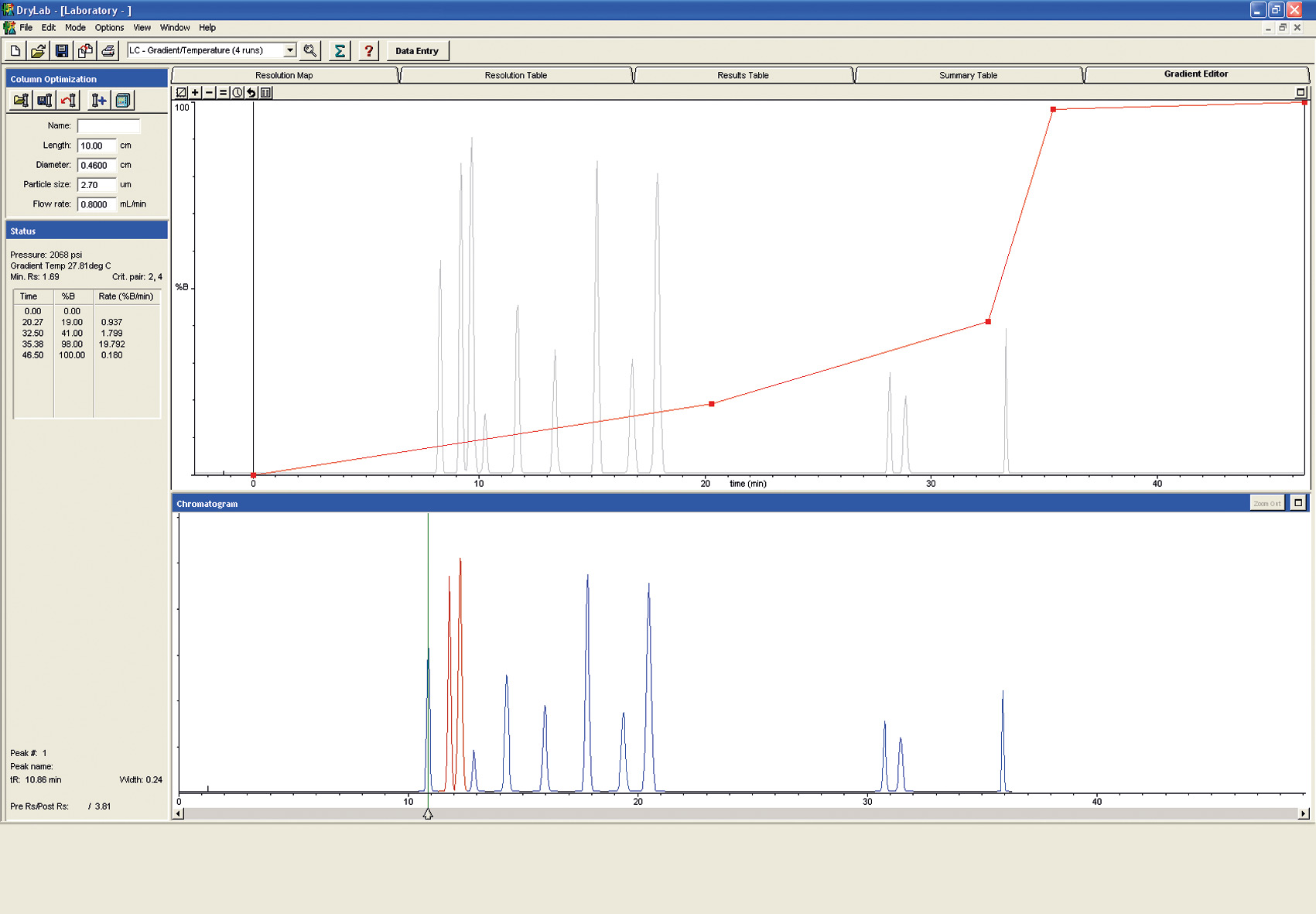 Figure 7: Gradient Editor
Figure 7: Gradient Editor
Overnight, these experiments can be run automatically and unattended using the Shimadzu LabSolution software.
Over 30 years of solvophobic theory [1] makes the continuous change in reversed-phase chromatography selectivity understandable. Retention forces are primarily based on the highly organized water structure and on the desire of water to reduce the cavity interfaces towards nonpolar molecules. Lipophobicity of water can be reduced by dilution with the strong eluent: MeOH or ACN.
The following example of an optimization of a separation of sulfonamides and additional drugs underlines the power of the DryLab2010 and Nexera combination.
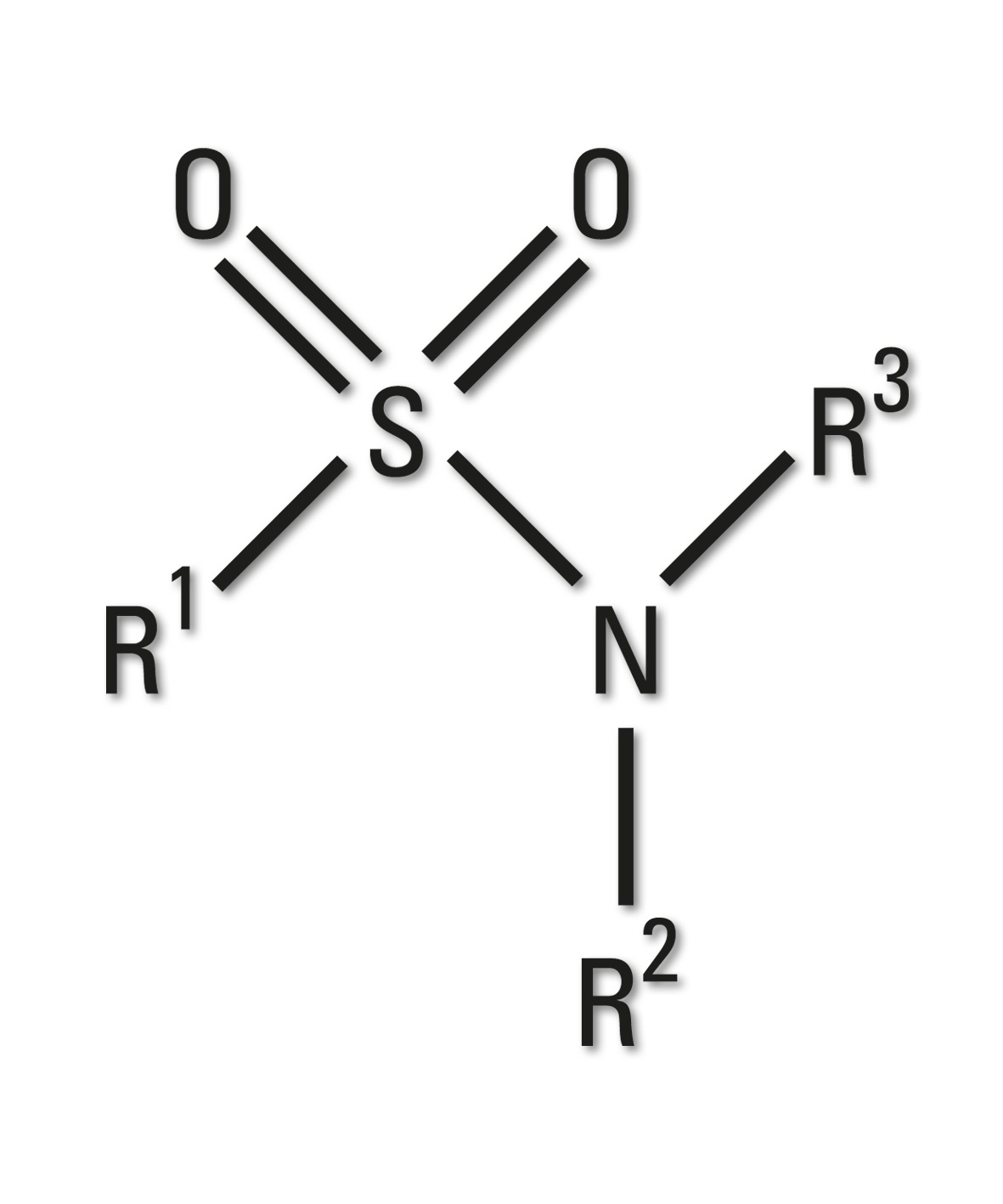 Figure 8: Sulfonamide group
Figure 8: Sulfonamide group
Sulfonamides are the basis of several groups of drugs. The original antibacterial sulfonamides are synthetic antimicrobial agents containing the sulfonamide group.
Standard configuration, prominence with LPGE, column oven and PDA detector.
Chemicals:
A: Buffer 25 mM phosphoric acid
B: Acetonitril
C: Buffer 25 mM Sodium Dihydrogen Phosphate
D: Methanol
Column: Gemini-NX® Phenomenex (150 x 4.6 mm, 3 µm)
Flow rate: 1.00 mL
Wavelength: 280 nm
Temperature: 40 °Celsius
Test mixture including following substances (see table 1).
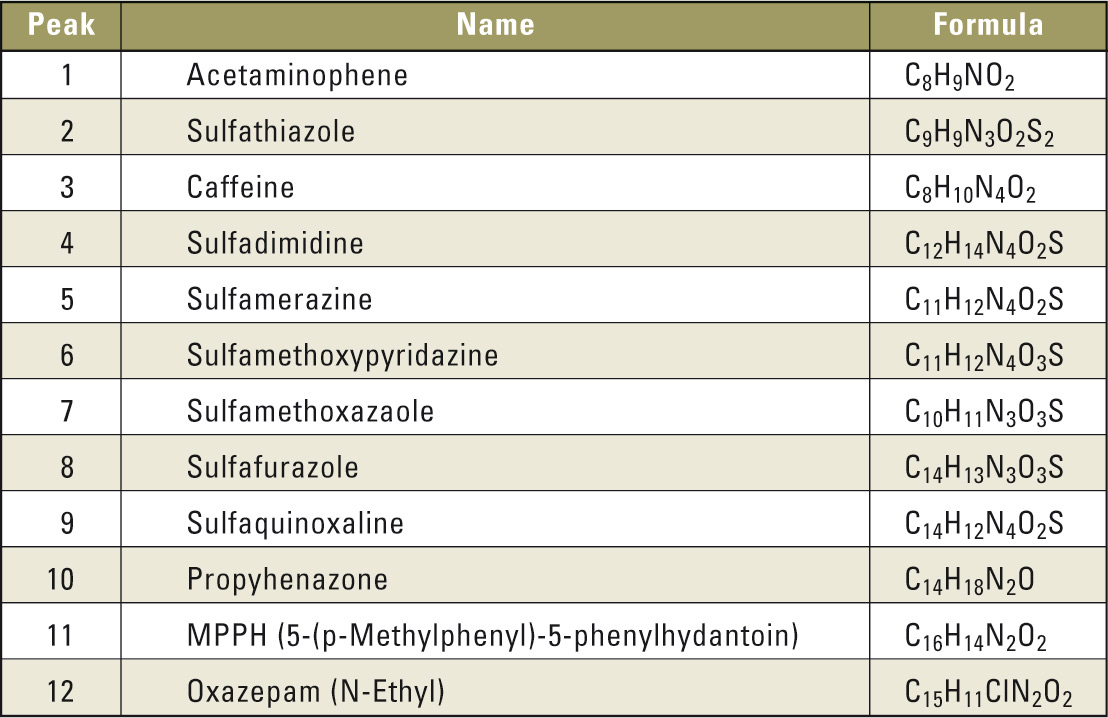 Table 1: List of compounds
Table 1: List of compounds
Optimization Step with DryLab 2010 3D Cube
Optimization on Nexera and Shim-Pack XR column with PDA detector
Flow rate: 1.30 mL/min
Temperature: 48 °Celsius
Wavelength: 280 nm
Column: Shim-pack XR-ODS III (75 x 2 x 1.6 µ)
Buffer A: 0.05 mol/L, pH = 2.3 Potassium dihydrogen phosphate (KH2PO4)
Buffer B: 50 % Methanol 50 % Acetonitril
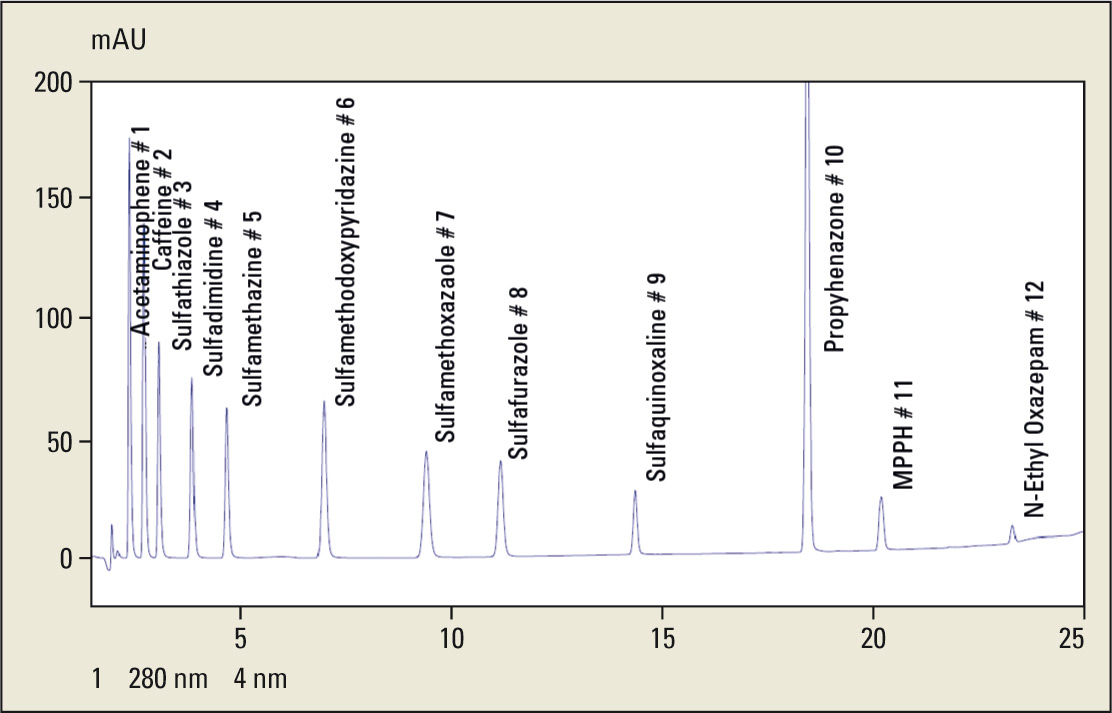 Figure 9: Chromatogram of scouting run
Figure 9: Chromatogram of scouting run
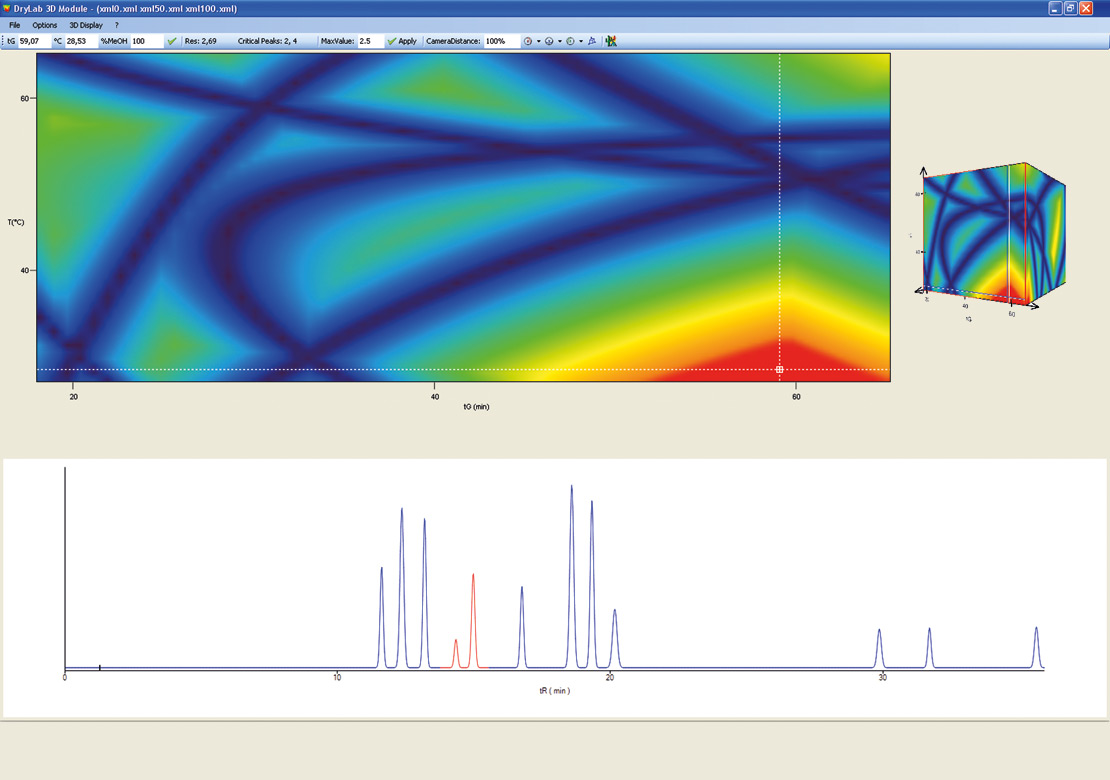 Figure 10: 3D Cube from DryLab®2010
Figure 10: 3D Cube from DryLab®2010
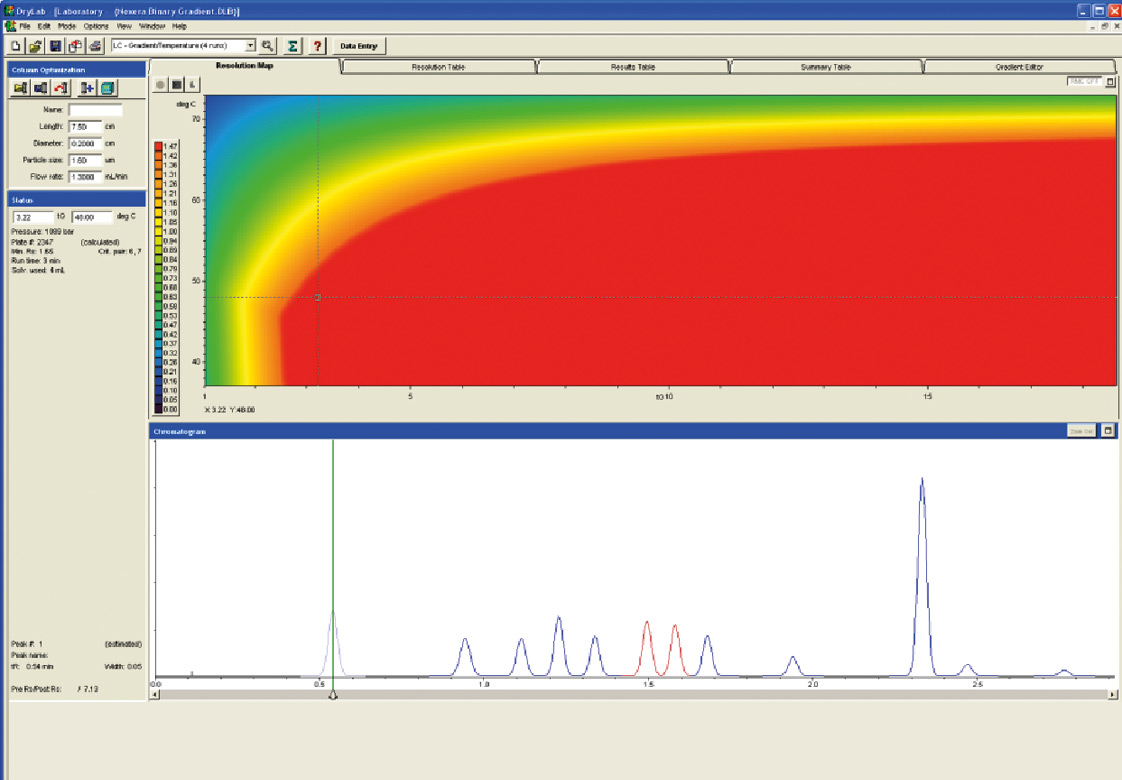 Figure 11: Prediction and Analytical Conditions with Shimadzu Nexera UHPLC
Figure 11: Prediction and Analytical Conditions with Shimadzu Nexera UHPLC
Conclusion
With the support of the Molnar Institute and the DryLab 2010 software the group of drugs was separated from starting conditions with a conventional HPLC and a runtime of more than 30 min by switching to a modern column, here the Shim-pack XR ODS III (75 x 2 x 1.6 µ) and the UHPLC System Nexera, with binary gradient to a runtime of less than three minutes. The critical peak pair (peak 6 and peak 7) has a resolution of 1.65; the pressure on the system is 1089 bar.
Quality by Design
The Quality by Design (QbD) concept was outlined by quality expert Joseph Juran (1904-2008), stating that quality can be planned, and that most quality problems relate to the way it was planned originally.
The ideal state for pharmaceutical manufacturing in the 21st century mean:
- understanding the product and its production processes
- understanding the methodologies (HPLC, etc.) which generate data to support the product quality.
- Solvophobic Theory Cs. Horvath, W. Melander, I.Molnàr J. Chromatogr. 125 (1976) 129.
- Aspects of the “Design Space” in high pressure liquid chromato-graphy method development; I.Molnàr; H.-J. Rieger, K. E: Monks J. Chromatogr. A, 1217 (2010) 3193-3200
Molnár-Institute for applied chromatography
Founded in 1981, the Institute brings 30 years of experience in high performance liquid chromatography (HPLC). Dr. Imre Molnár, former coworker of Prof. Csaba Horváth from Yale and of Lloyd Snyder and John Dolan of LCResources USA, is a well known expert in HPLC method development.
The Molnár-Institute plays a small but essential role in the improvement of worldwide healthcare, ensuring safe products in pharmaceutical, life science- and food industries, while supporting research and development at universities. The Molnár-Institute continuously serves companies all over the world in successfully designing and shaping HPLC method conditions. By offering software solutions, courses and development services, the Molnár-Institute defines its mission in applying modern software tools such as DryLab2010 to increase efficiency in HPLC laborato-ries and to help to find the best separation as soon as possible.