A global traveler
Spectroscopic methods in polymer packaging analysis
The apple is one of the most widely cultivated tree fruits. In 2008, 64 million tons of apples were grown worldwide. China produced 27.5 million tons, over 40 % of this total. The US is the second leading producer, with approx. 7 % of world production.
Research suggests that apples may reduce the risk of colon cancer, prostate cancer and lung cancer. They are a rich source of antioxidant compounds. They may also help to combat heart diseases.
Apples are grown and consumed worldwide. They are global travellers. In order to get them in good shape and best condition for markets and consumers they have to be treated well and need to enjoy a safe trip.
Preparation and packing processes are automated. Modern machines sort the fruits for size and quality and pack them into a carton. Important criteria for the packing are:
- the contents must be of the same appearance – the apples must have the same size and color
- the packaging must protect the apples from impact
- the packaging must be new, clean and with printed marks and information
The carton is regarded as being the best transportation device for apples. The insert has been designed particularly to shelter and also stabilize the fruits. The insert shown here has two areas: a stiff one framing the fruits (Figure 1 and 2), and a soft and smooth area (Figure 1 and 3). In the following text the polymer foil insert of a carton transport packaging is analyzed.
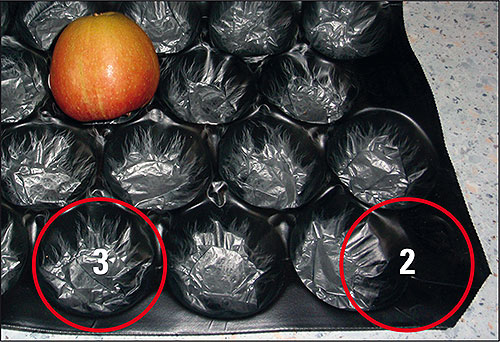 Figure 1: An apple in a polymer foil/insert with specific dimensions for the transport of fruits. The red circles mark the two analysis areas shown in figure 2 and 3.
Figure 1: An apple in a polymer foil/insert with specific dimensions for the transport of fruits. The red circles mark the two analysis areas shown in figure 2 and 3.
 Figure 2: Surface of the black colored foil part, a polymer which is rough and stiff. It is from the edge of the insert shown in figure 1.
Figure 2: Surface of the black colored foil part, a polymer which is rough and stiff. It is from the edge of the insert shown in figure 1.
 Figure 3: Surface of the silver-gray colored, very thin soft foil part
Figure 3: Surface of the silver-gray colored, very thin soft foil part
The following application shows how FTIR, EDX and ICP techniques complement each other. Whereas FTIR and EDX do not destroy the samples, ICP needs sample preparation allowing better detection limits regarding the elements in the sample.
Modern analysis techniques
The material was analyzed with molecular spectroscopy represented by FTIR and elemental analysis through EDX and ICP technique. A benefit of FTIR and EDX is non-destruction of the sample during the analysis process. The advantage of the ICP is the multi-elemental detection in one measurement, independent of the weight of the elements of interest.
Analysis with FTIR IRAffinity-1
The insert sample was analyzed without chemical treatment. Since the material in figure 3 was very thin, transmission mode measurement was possible. Figure 4 shows the results. The infrared spectrum shows a polypropylene spectrum as expected based on the PP sign printed on the insert. Of interest is the view of the baseline from this measurement showing a strong slope.
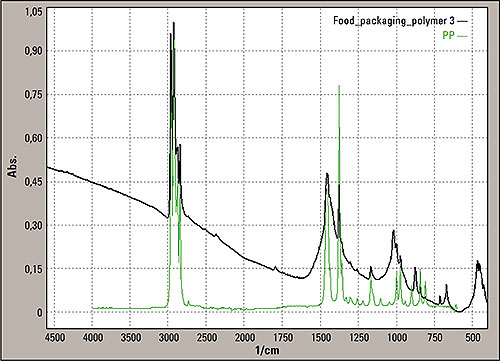 Figure 4: Transmission mode spectrum from the sample area three of the insert (black line), green is the typical polypropylene peak from the spectral library
Figure 4: Transmission mode spectrum from the sample area three of the insert (black line), green is the typical polypropylene peak from the spectral library
The slope in the baseline of the insert is comparable with the Christiansen effect of particles in a KBr pellet (potassium bromide) known for the transmission measurement. Knowing this, it is to be expected that some solid particles are part of the foil construction. The single reflection “Silver Gate” unit equipped with a ZnSe crystal (zinc selenide) was used for surface measurements of area 2 in figure 1. The material is too thick for transmission mode analysis.
The measurement technique allows a penetration of 2 μm of the beam into the sample surface. The spectrum is shown in figure 5. For the analysis of the unknown spectra, the Shimadzu and commercial libraries such as Sadtler were used to identify the infrared spectra.
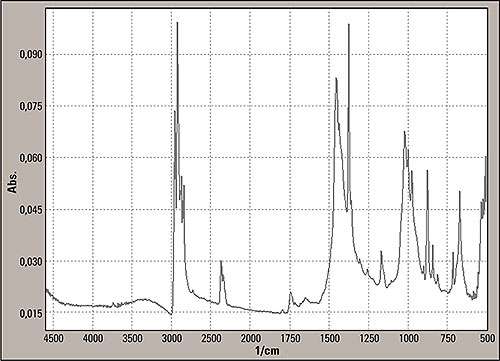 Figure 5: Reflection mode spectrum from the insert part surface, marked with two in figure 1, consisting mainly of polypropylene
Figure 5: Reflection mode spectrum from the insert part surface, marked with two in figure 1, consisting mainly of polypropylene
In these libraries the base material polypropylene (PP) was found. Next major deviations were related to supra plast material and a polysilicate (reinforcing filler silver white fibres with black inclusions) being found in the Sadtler Hummel library. The presence of this filler is correct because the silicate contained can be the cause of the tremendous slope of the baseline of the transmission mode. The silicate particles generate stray light phenomena.
Instrumentation:
Instrument: IRAffinity-1, Shimadzu
Accessory: Silver Gate, ZnSe crystal, Specac
Libraries: Shimadzu, Sadtler – BioRad Devision
Analysis with EDX-720
As some polysilicate and filler were found, it was interesting to research the type of filler used for the foil. Based on the match the library first suggested an asbestos mineral based on magnesium (Mg), iron (Fe) and silicon (Si). However, fillers are not based on these materials. EDX quickly helped to identify the main elements which were bases of the mineral/filler. Calcium (Ca), silicon (Si) and iron (Fe) were found in high concentrations. The Ca signal was extraordinary high. The light elements were not analyzed.
Analysis with ICP-9000
The presence of silicon in the samples was found by FTIR and EDX, so the samples were decomposed by a solution of 5 ml HF and 10 ml HNO3 for the ICP-analysis.
Using this decomposition medium the silicon was completely dissolved. The sample was measured with the Shimadzu ICPE-9000. The result is shown in the following table. A double measurement from each sample was performed as well as a blank and a control standard to proof the accuracy of the method.
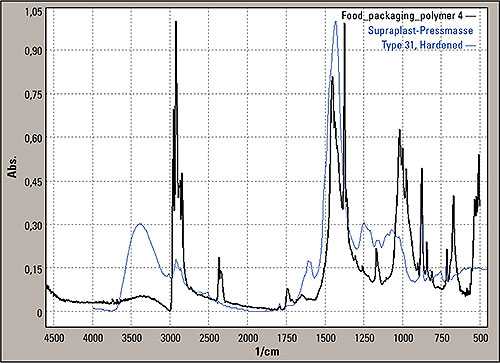 Figure 6: Search result for the signals differing from PP, Hummel/Sadtler Polymers, SUPRAPLAST-PRESSMASSE TYPE 31, HARDENED (blue line)
Figure 6: Search result for the signals differing from PP, Hummel/Sadtler Polymers, SUPRAPLAST-PRESSMASSE TYPE 31, HARDENED (blue line)
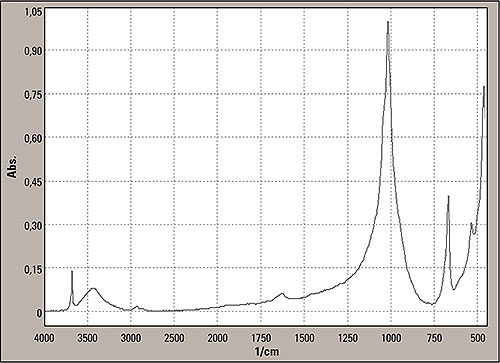 Figure 7: Last signals search in the inorganics library showed the following: SILVER-WHITE FIBERS WITH BLACK INCLUSIONS, Chemical Description = POLYSILICATE, Comments, Use: REINFORCING FILLER
Figure 7: Last signals search in the inorganics library showed the following: SILVER-WHITE FIBERS WITH BLACK INCLUSIONS, Chemical Description = POLYSILICATE, Comments, Use: REINFORCING FILLER
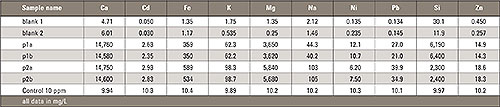 Table 1: Measurement results of polypropylene after microwave digestion [all values in mg/L]
Table 1: Measurement results of polypropylene after microwave digestion [all values in mg/L]
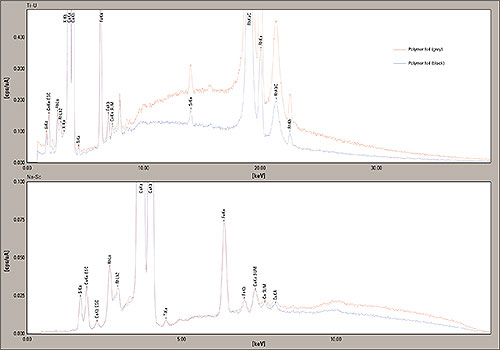 Figure 8: EDX-Analysis of two Polymer samples (apple film grey and apple film black), measured from 0 -40 keV and 0 -15 keV, the peaks of Calcium (Ca), Silicon (Si), Iron (Fe) and Potassium (K) are clearly visible
Figure 8: EDX-Analysis of two Polymer samples (apple film grey and apple film black), measured from 0 -40 keV and 0 -15 keV, the peaks of Calcium (Ca), Silicon (Si), Iron (Fe) and Potassium (K) are clearly visible
Conclusion
The polymer foil contains components made of materials other than polypropylene. Based on the characteristics of the material and the target usage, the PP has been treated with some fillers. The fillers are usually low-priced minerals such as talc, sand, calcium carbonate and hematite. The analysis time with all three instruments was less than 15 minutes.
Talc: Mg3Si4O10(OH)2
Sand/quartz: SiO2
Feldspar: KAl Si3O8
Calcium carbonate: CaCO3
Hematite: Fe2O3