Completely pure
TOC analysis for cleaning validation in the pharmaceutical industry
When manufacturing pharmaceuticals, highest purity and careful handling of substances and active ingredients are essential. It is especially important to remove all process residues from production effectively, because only a well cleaned plant prevents contamination and adulteration of the drug produced, thereby contributing to patient safety.
In 1988, for example, 200 million doses of a cholesterol-lowering drug had to be recalled – they were contaminated with pesticides. It is assumed that there was a cross-contamination, as solvent drums from the pesticide production were re-used in the manufacturing of the medication. For finishing, the active ingredient was delivered to another facility where normally no pesticides are manufactured, and whole product batches were contaminated. At that time, the drug manufacturer neither carried out adequate controls, nor validated cleaning procedures.
Regulatory requirements
As a result, key regulatory requirements for cleaning were specified in 1993 in the “Guide to Inspections – Validation of Cleaning Processes (7/93)” of the FDA (American Food and Drug Administration). Documented evidence is now required that demonstrates an approved cleaning process which consistently reduces active pharmaceutical ingredients (API), process residues, detergents and microbial contaminants on product contact surfaces of manufacturing equipment to an acceptable level for the processing of pharmaceuticals. This is particularly important in the production of compounds in batch processes, as the plant is used for various substances and contamination of the downstream products must be avoided.
When a compound batch leaves the facility, the equipment used, e.g. reactors or fermenters is cleaned before the next batch can be produced. The cleaning process to be used is usually strictly specified, and is then evaluated analytically and documented. A sample from the plant is examined for certain parameters. If a defined limit value is not exceeded, the system is regarded as having been cleaned and may be used again. This process is called cleaning validation. The cleaning method used has a great influence on the type of sampling technique required.
Cleaning procedure ‘Clean in Place’
CIP (clean in place) cleaning occurs automatically without dismantling of the system. This means that the system must be build according to CIP design. This includes the use of rinsing heads, collection tanks, avoidance of dead spaces, and recycling possibilities of the cleaning agent. CIP cleaning is very effective because time and temperature, as well as cleaning agent and solvent consumption are optimized. Automatic cleaning also enables a standardized and easy to validate procedure. In the case of CIP cleaning, the rinsing liquid of the last rinse cycle (final rinse) is taken as a sample and analyzed. This is a particularly simple, easily automated and fast method.
Cleaning procedure ‘Clean out of Place’
For COP (clean out of place) cleaning, the system must be disassembled and the components cleaned individually. This procedure is very time consuming and labor intensive. Due to the individual cleaning, this procedure cannot be standardized. However, the lower cost of the system and the possibility of visual assessment are beneficial.
In case of COP-cleaning, wiper testing (swab) is used to sample visible residues (figure 1). These include coatings, crusts, caking in angles and corners and substances that are particularly difficult to dissolve. The swab used can be extracted in a solvent and the extraction solution is then examined analytically. If water is used as an extraction solvent, TOC determination is used for subsequent analysis. Alternatively, the swab can also be analyzed directly (using a carbon-free swab) with a TOC solids module.
 Figure 1: Shimadzu Easy Wiper Swab
Figure 1: Shimadzu Easy Wiper Swab
Applicable analysis methods
Several analytical methods are available to verify successful cleaning, but they differ in sensitivity and specificity. Common methods are shown in table 1.
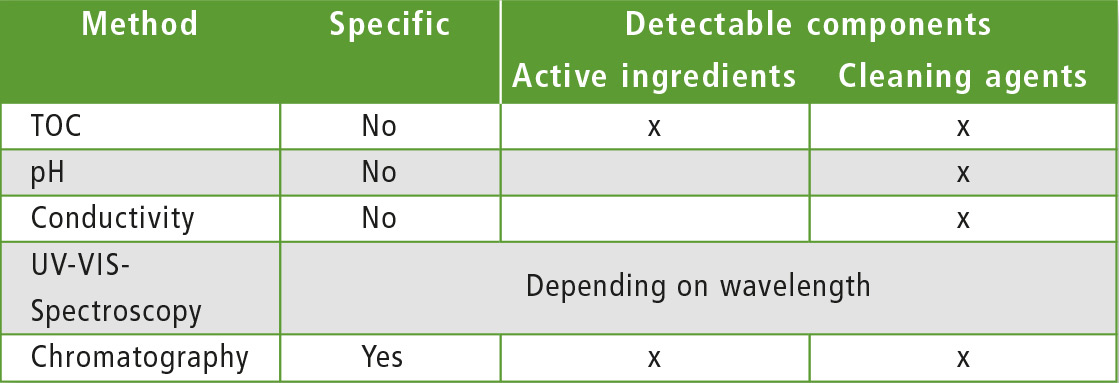 Table 1: Methods of analysis
Table 1: Methods of analysis
Using specific methods such as HPLC or GC, agents and detergents can be selectively detected. However, it is possible in some chemical cleaning processes that initially existing components can no longer be detected due to decomposition reactions. If acids or alkalis are used as additional cleaning agents, non-specific parameters such as pH or conductivity are often used for detection. However, it is not possible to detect active ingredients and excipients in this way.
To determine quickly whether the concentration of active substances and residues of cleaning agents and auxiliary substances is low enough after cleaning, the sum parameter TOC can be applied.
TOC analysis in cleaning validation
TOC (Total Organic Carbon) analysis captures all carbon from organic compounds in one analysis and is therefore particularly suitable for determining contamination by organic components. Carbon content of the sample is oxidized to CO2 and analyzed with an NDIR (non-dispersive IR) detector.
The TOC value reflects the total organic contamination caused by the precursor, the additives used and the remaining cleaning agents. Aqueous samples (final rinse or swabs eluted in ultrapure water) can thus be analyzed quickly and easily (analysis time: approx. 4 min).
Precondition required is good water solubility of all substances. For water-insoluble substances, direct combustion of the carbon-free swabs is recommended. Table 2 gives examples of recovery rates of various water-soluble and water-insoluble substances in the sample types mentioned.
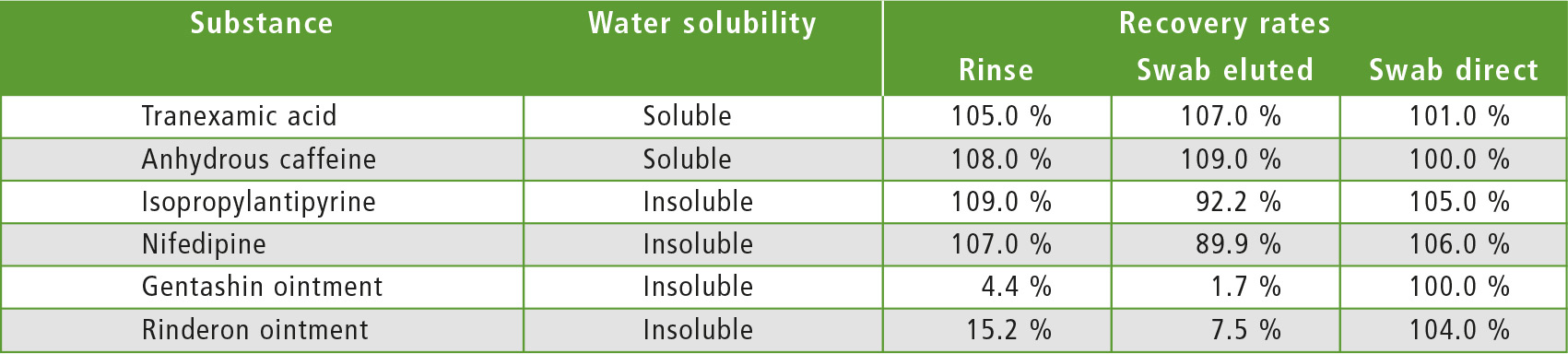 Figure 2: TOC-L with SSM-5000A
Figure 2: TOC-L with SSM-5000A
Two methods, one device
For cleaning validation, a mixture of swab method and final rinse method is usually used. This allows the entire plant as well as special critical locations to be examined with maximum sensitivity. Rinse water and swab samples obtained by extraction and by direct combustion can be tested using a single TOC analyzer.
Modern analyzers such as Shimadzu’s TOC-L series handle the preparation of liquid samples automatically (acidifying and sparging). With a high volume of samples, this can be done in an autosampler to save time.
The systems operate with a highly efficient platinum catalyst at 680 °C combustion temperature. A special injection unit enables automatic dilution of the sample if the calibration range is exceeded. Standards are also automatically diluted to create calibration curves at equidistant concentration intervals.
For direct combustion of swab samples, the analyzer is combined with the SSM-5000A solid sampling module (figure 2). Swabs are combusted directly in an oxygen environment at 900 °C with a catalyst. Quantification of the CO2 combustion product subsequently enables determination of TOC.
 Table 2: Recovery rates for different substances and sampling types
Table 2: Recovery rates for different substances and sampling types
Conclusion
In the pharmaceutical industry, various cleaning methods are used, for example “clean in place” or “clean out of place”, whose validation is bound to certain techniques. TOC analysis is especially suitable for determination of contamination caused by organic compounds. This is done with the final rinse method or the swab method, each with advantages and disadvantages. Both are therefore used in cleaning validation. With the TOC-L series systems, cleaning of production plants in the pharmaceutical industry can be documented quickly and easily using both methods.