Small but powerful!
Small columns accelerate conventional USP methods for medical use and save solvents, time, and money
 Shimadzu Nexera X2 SPD-M30A
Shimadzu Nexera X2 SPD-M30A
In order to increase productivity and efficiency in laboratories, high-throughput analyses have become very important in recent years. Especially in the pharmaceutical industry, fast methods are an advantage as this industry faces many challenges: cost-cutting measures in the European and American healthcare sector limit growth and profit potential. The development of new drugs has become increasingly difficult and expensive. [1, 2]
Therefore, it is important to reduce costs in other ways. For example, by performing analyses as quickly as possible and more economically. This can be achieved in liquid chromatography (LC) by using a smaller column. Using examples from the official pharmacopoeia of the United States Pharmacopeia (USP), the possibilities were investigated. The pharmacopoeia contains general chapters on tests as well as individual monographs. A monograph consists of tests on active ingredients and their specifications.
In particular, the chapter “Chromatography” [3] (USP 621) is paramount, since the pharmacopoeia has been adapted for the use of smaller column dimensions and now allows, to a certain extent, the modification of HPLC and GC parameters in order to obtain faster methods.
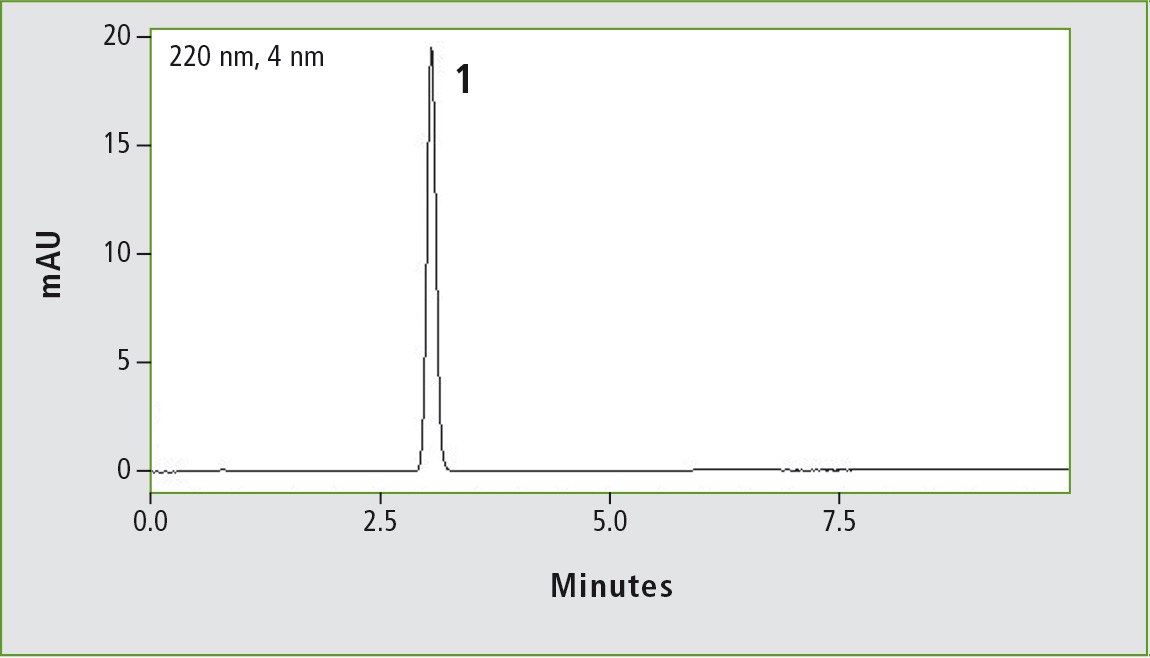 Figure 1a: Chromatogram of acetaminophen (0.01 mg/mL) obtained through the original USP method. Column: (a) Shim-pack GIS C18 (250 x 4.0 mm, 10 µm).
Figure 1a: Chromatogram of acetaminophen (0.01 mg/mL) obtained through the original USP method. Column: (a) Shim-pack GIS C18 (250 x 4.0 mm, 10 µm).
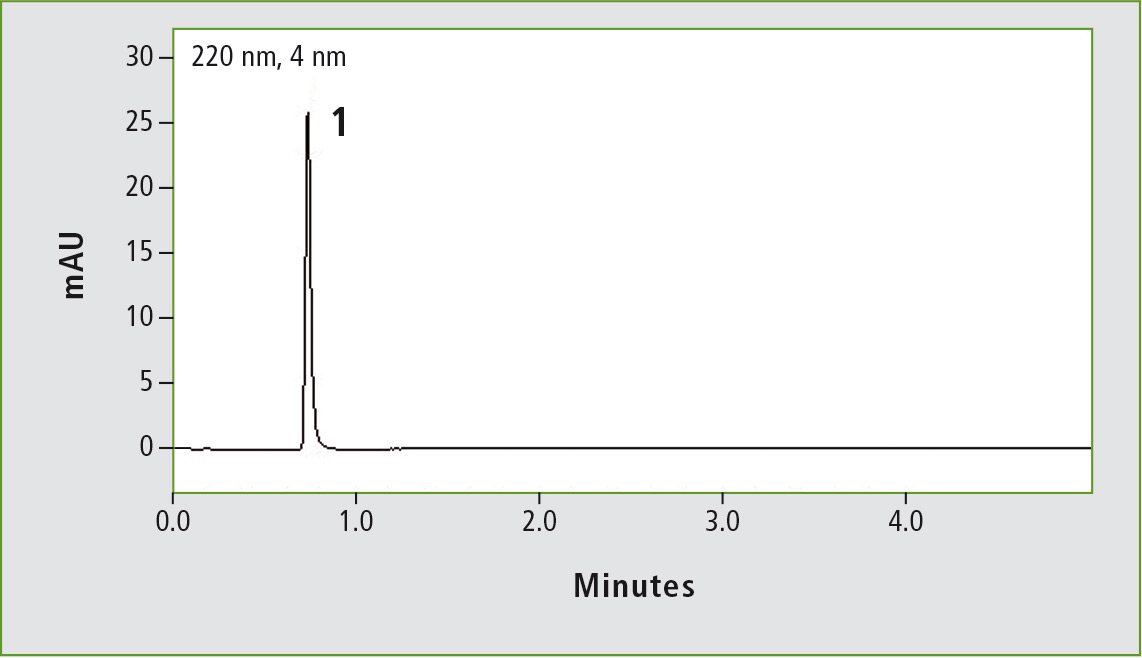 Figure 1b: Chromatogram of acetaminophen (0.01 mg/mL) obtained through the accelerated USP method. Column: (b) Shim-pack GIS C18 (100 x 3.0 mm, 3 µm).
Figure 1b: Chromatogram of acetaminophen (0.01 mg/mL) obtained through the accelerated USP method. Column: (b) Shim-pack GIS C18 (100 x 3.0 mm, 3 µm).
Chapter USP <621> is focused on chromatography
This chapter of the USP is of a general scope and contains information on all types of chromatography, such as gas, thin-layer, paper, and liquid chromatography. The principles of chromatography, including the basics as well as the equipment, and procedures used for the analyses are listed here. In addition, all important parameters which are necessary when analyzing a chromatogram are explained.
These USP application parameters, such as retention time, resolution, or plates, have certain target values, which must be met by applications modified from the original method. Table 1 shows the parameters, which may be changed in reference to USP 621. Additionally, the permitted ranges within these LC parameters are listed.
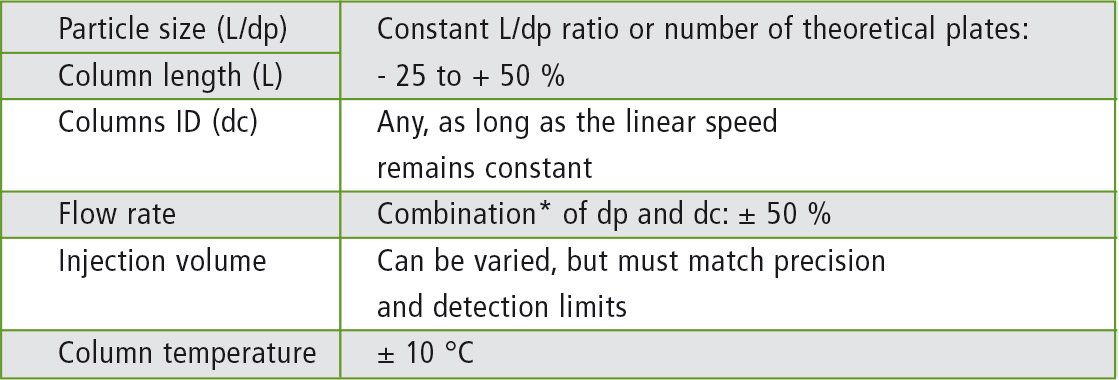 Table 1: Permitted range of changes of the LC parameters in accordance with USP <621>
Table 1: Permitted range of changes of the LC parameters in accordance with USP <621>
*F2 = F1 x [(dc22 x dp1)/(dc12 x dp2)] · F1 and F2 are the flow rates of the original and the modified method; dc1 and dc2 are the column diameters according to these methods; dc1 and dc2 are the particle size.
In the following, isocratic, conventional analyses of different medicines were modified while observing the permitted adaptation criteria of the USP. As shown in the example, the investigations of acetaminophen, ibuprofen, and glibenclamide could be carried out faster than described in the original monograph.
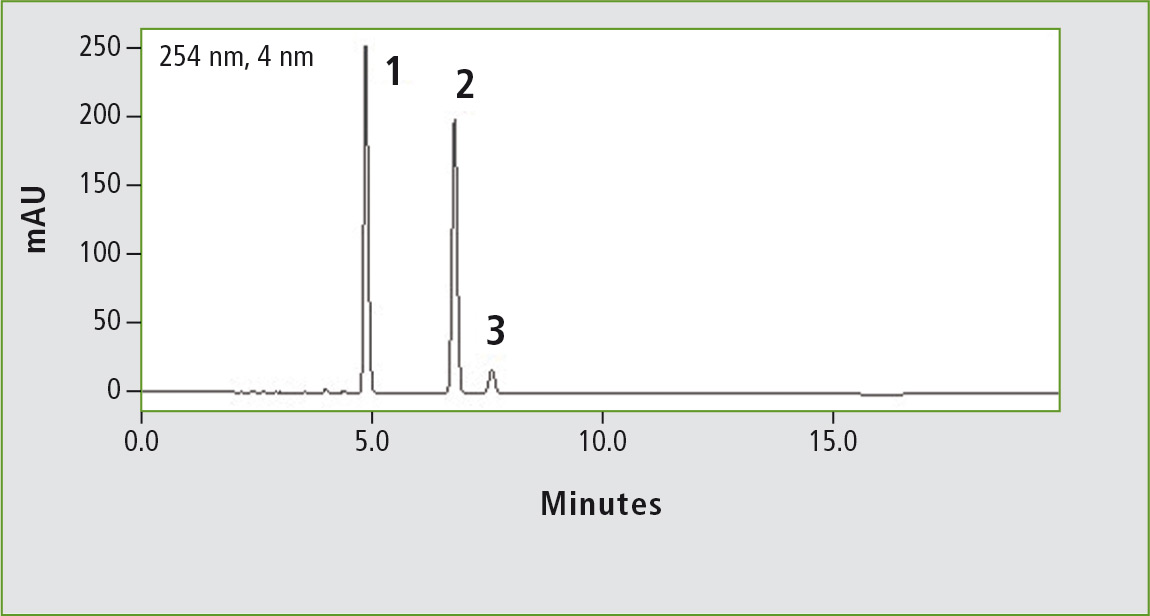 Figure 2a: Chromatogram of ibuprofen (peak 1) (12 mg/mL) obtained through the original USP method. Column: (a) Shim-pack GIST C18 (250 x 4.6 mm, 5 µm). Peak 2: vale-rophenone (0.35 mg/mL), peak 3: 4 isobutylacetophenone (0.012 mg/mL).
Figure 2a: Chromatogram of ibuprofen (peak 1) (12 mg/mL) obtained through the original USP method. Column: (a) Shim-pack GIST C18 (250 x 4.6 mm, 5 µm). Peak 2: vale-rophenone (0.35 mg/mL), peak 3: 4 isobutylacetophenone (0.012 mg/mL).
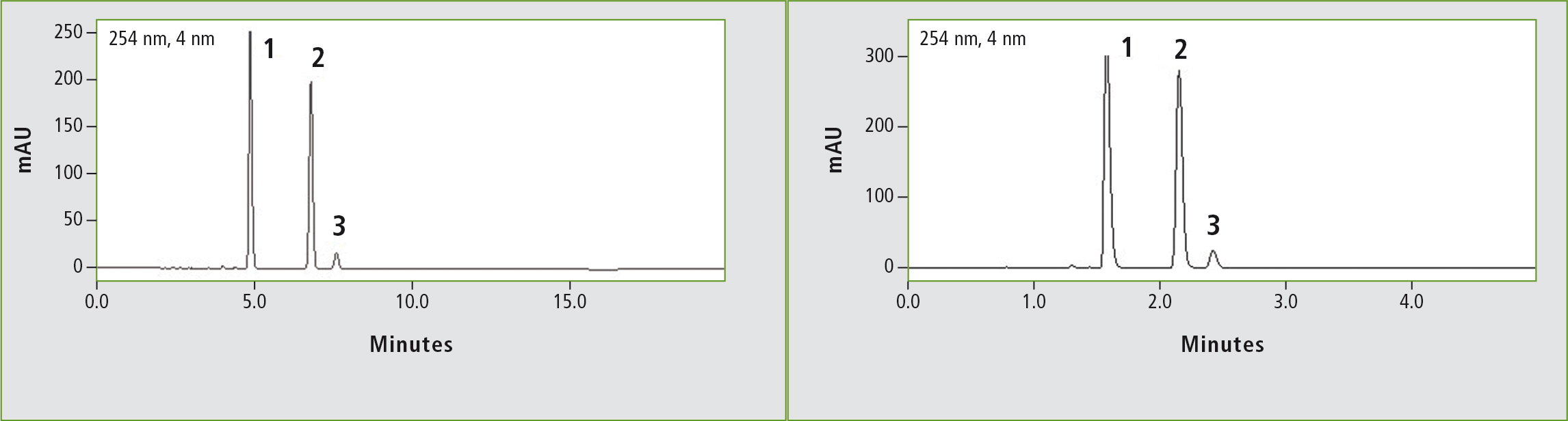 Figure 2b: Chromatogram of ibuprofen (peak 1) (12 mg/mL) obtained through the accelerated USP method. Column: (b) Shim-pack GIST C18 (100 x 2.1 mm, 2 µm). Peak 2: Valerophenone (0.35 mg/mL), peak 3: 4 isobutylacetophenone (0.012 mg/mL).
Figure 2b: Chromatogram of ibuprofen (peak 1) (12 mg/mL) obtained through the accelerated USP method. Column: (b) Shim-pack GIST C18 (100 x 2.1 mm, 2 µm). Peak 2: Valerophenone (0.35 mg/mL), peak 3: 4 isobutylacetophenone (0.012 mg/mL).
Advantages of shorter analysis times – achieved with smaller column dimensions
When replacing standard columns (250 or 150 x 4.6 mm) with smaller equivalents (e.g. 100 x 3 mm) packed with 2 – 3 µm instead of 5 µm particle sizes, different effects occur: the efficiency of the separation performance increases, resulting in narrower signals, and the analytes elute faster from the column. At the same time, the flow rate must be reduced, otherwise the pressure increase in the system would be too high. This provides two major improvements: a much shorter analysis time, which results in a higher sample throughput and a higher workload of the device, as well as a lower consumption of solvents. With the saving of time and solvents, the down-scaling offers two advantages, which in turn greatly reduce expenses.
Adaptation of USP methods in accordance with the guidelines
The permitted ranges within which the analytical conditions may be modified can be found in USP chapter <621> Chromatography. By changing the analytical conditions in accordance with the guidelines, the analysis time can be significantly shortened. Here, a shorter column with a smaller inner diameter was selected and the flow rate was correspondingly reduced in order to maintain the linear velocity. In order to maintain the resolution of the separation, the length and the particle size of the column may be modified as long as the ratio of the column length (L) to the particle size (dp) remains within the specified range (permissible range: – 25 % to + 50 %).
 Shimadzu LC-2040C 3D
Shimadzu LC-2040C 3D
Reduction of analysis time
Various medicines, which can be identified by LC methods described in the USP, have been tested. The original methods were modified by using smaller columns and lower flow rates according to USP guidelines. All analyses were measured under isocratic conditions.
Used medicines
To demonstrate how much faster this new approach is, compared to the conventional methods of the pharmacopoeia, three different medicine analyses were chosen:
- Acetaminophen, better known as paracetamol, is a medication to treat fever and pain. [4]
- Ibuprofen is a non-steroidal anti-inflammatory drug. The drug is used to relieve fever, inflammation, and pain. [5]
- Glibenclamide acts as an antidiabetic agent. The active substance belongs chemically to the group of the sulphonylureas. The drug increases insulin release from the pancreas in order to lower blood sugar. [6]
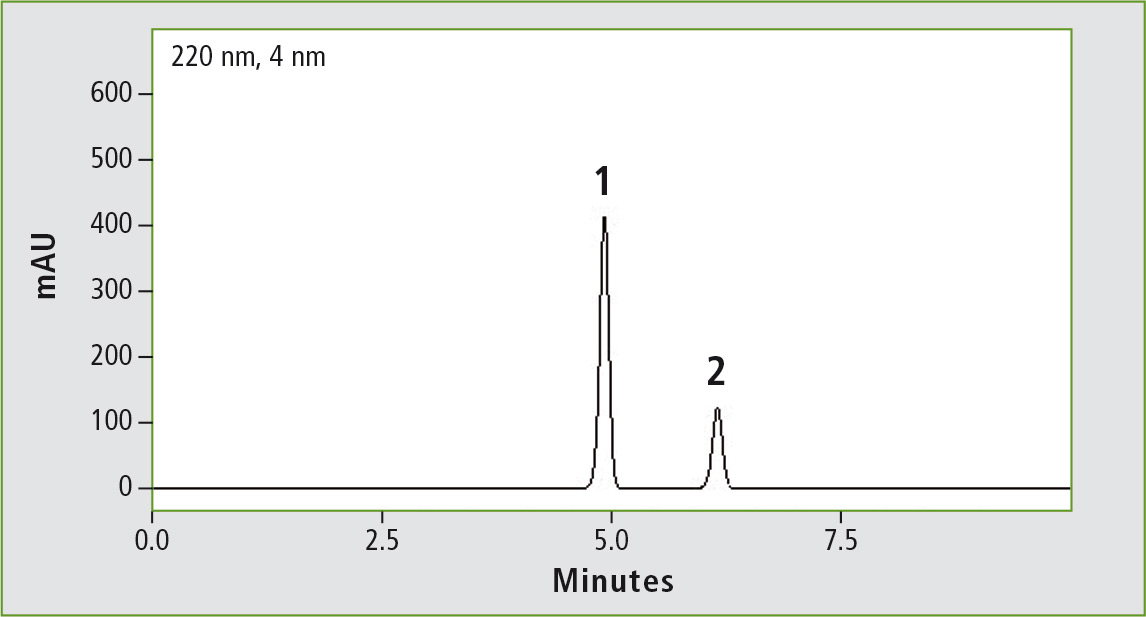 Figure 3a: Chromatogram of glibenclamide (peak 1) (0.44 mg/mL) obtained through the original USP method. Column: (a) Shim-pack GIST C8 (250 x 4.6 mm, 5 µm). Peak 2: progesterone (0.2 mg/mL).
Figure 3a: Chromatogram of glibenclamide (peak 1) (0.44 mg/mL) obtained through the original USP method. Column: (a) Shim-pack GIST C8 (250 x 4.6 mm, 5 µm). Peak 2: progesterone (0.2 mg/mL).
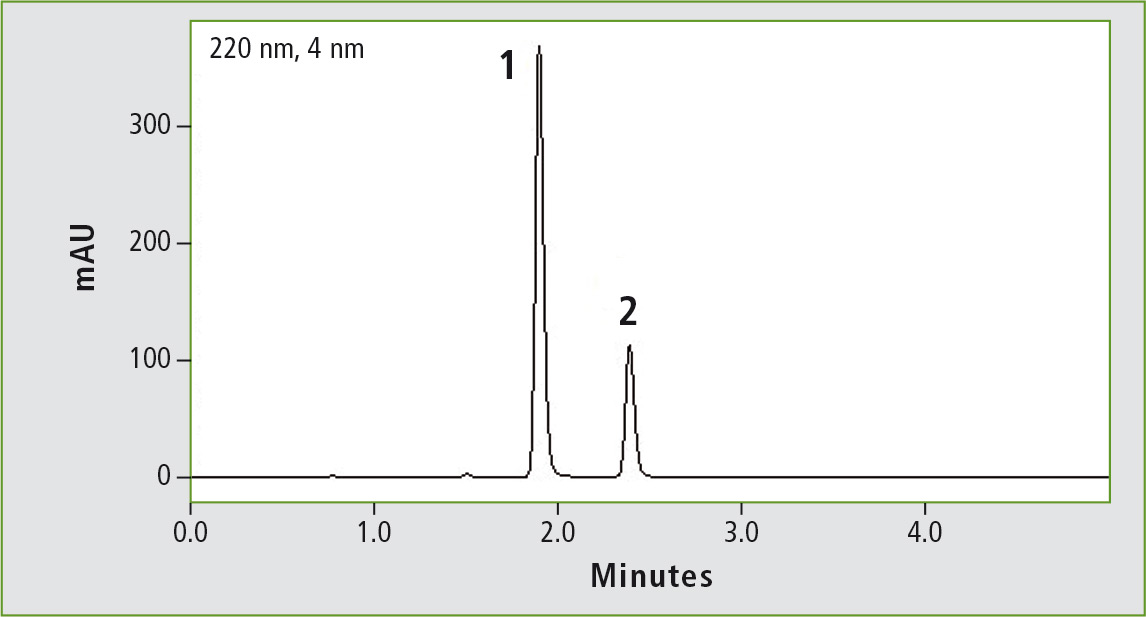 Figure 3b: Chromatogram of glibenclamide (peak 1) (0.44 mg/mL) obtained through the accelerated USP method. Column: (b) Shim-pack GIST C8 (100 x 2.1 mm, 2 µm). Peak 2: progesterone (0.2 mg/mL).
Figure 3b: Chromatogram of glibenclamide (peak 1) (0.44 mg/mL) obtained through the accelerated USP method. Column: (b) Shim-pack GIST C8 (100 x 2.1 mm, 2 µm). Peak 2: progesterone (0.2 mg/mL).
Measurement parameters and methods
The measurement parameters as well as the methods are shown in Tables 2 and 3.
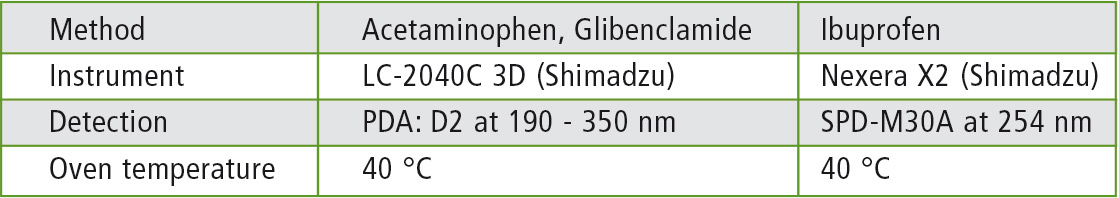 Table 2: Instruments and parameters used
Table 2: Instruments and parameters used
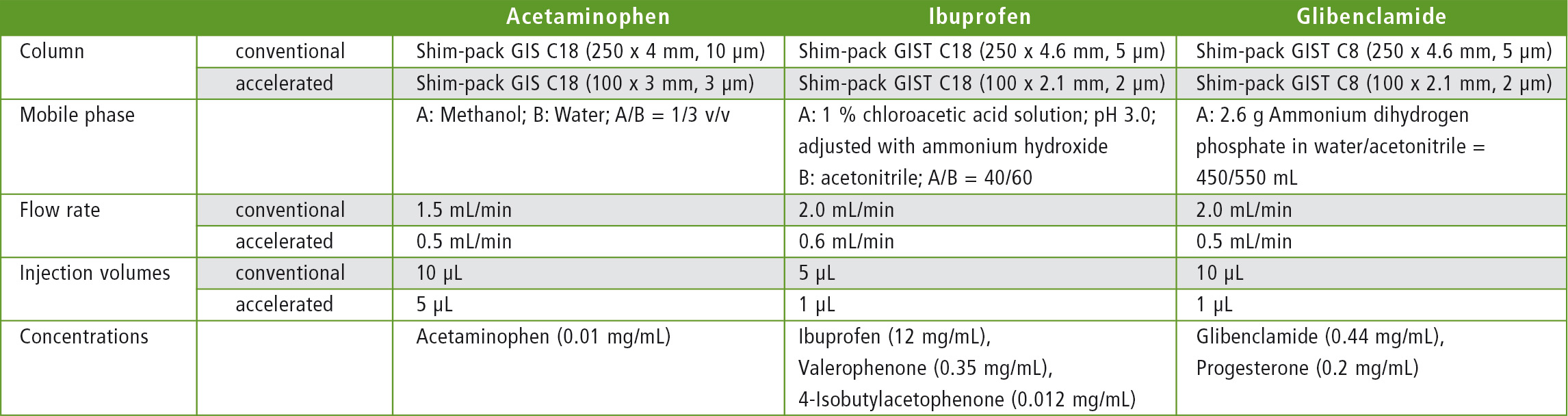 Table 3: Columns and method parameters used
Table 3: Columns and method parameters used
Results
The results are shown in figures 1 – 6 and in table 4. These indicate shorter retention times. The original USP methods have been greatly accelerated by changing the length, the internal diameter, and the particle size of the columns. The flow rate was adjusted to keep the linear velocity always constant. The retention times were thus shortened, resulting in time savings of 77 % for acetaminophen, 67 % in the analysis of ibuprofen and 61 % for glibenclamide. The solvent consumption of the three analyses was also significantly reduced: 67 % for acetaminophen, 70 % for ibuprofen, and 75 % for glibenclamide.
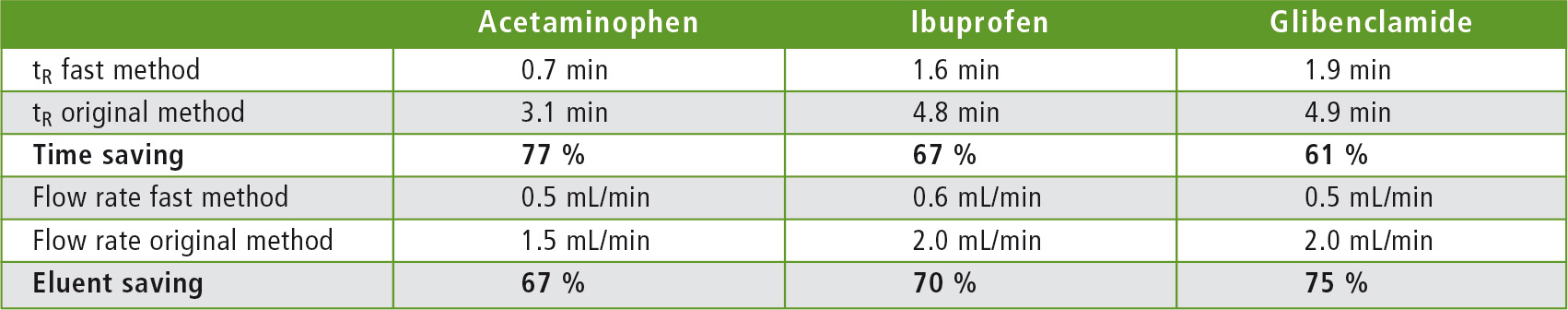 Table 4: Retention times and flow rates of the accelerated, as well as the original USP methods including calculated savings
Table 4: Retention times and flow rates of the accelerated, as well as the original USP methods including calculated savings
Conclusion
The USP methods for acetaminophen, ibuprofen, and glibenclamide have been significantly improved by using smaller columns compared to the original USP methods. All analysis times are shorter, and less solvents are consumed. As a result, the costs per analysis are significantly reduced. The method transfer from larger columns to small columns offers a lot of advantages altogether, hence the headline: small but powerful!
Literature
[1] Gehrke, von Haaren-Giebel, Branchenanalyse Pharmaindustrie, Study 305, Dezember 2015.
[2] Dohrmann, M., Biecheler, P., Hosseini, M., Nyctelius, H. (2012): Pharma’s fight for profit-ability. Lifecycle-based operating models.
[3] United States Pharmacopeia (USP) 39, <621> Chromatography, August 2016.
[4] Anderson B.J. Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth, 2008, 18(10), 915-21.
[5] Melzer, M.: Ibuprofen: Wirkung, Anwendung, Nebenwirkungen. Apotheken Umschau 2017.
[6] Davidson J.A., Scheen A.J., Howlett H.C. Tolerability profile of metformin/glibenclamide combination tablets (Glucovance): a new treatment for the management of type 2 diabetes mellitus. Drug Saf, 2004, 27(15), 1205-16.