Pesticides: killers of bee colonies
Ultra-sensitive and rapid assay of neonicotinoids, fipronil and some metabolites in honey by UHPLC-MS/MS

Neonicotinoids are a class of insecticides widely used to protect crop areas such as corn, canola and soybean as well as fruit and vegetables. These substances have an influence on the central nervous system of insects, causing paralysis and death. The first neonicotinoid, imidacloprid, was discovered by Shinzo Kagabu (Bayer CropScience, Japan). Their systemic distribution with high efficiency against sucking insects and long residual activity has made them very popular within the global pesticide market. Nowadays, there are roughly ten molecules classified as neonicotinoids (please see table 1).
Use of these compounds has recently become controversial as they are believed to be a cause of honeybees Colony Collapse Disorder (CCD). Since pollination is essential for agriculture, extensive studies have been conducted to evaluate the impact of neonicotinoids on bee health.
The European Food Safety Authority (EFSA) has identified risks to bees in the use of neonicotinoids. Three types of insecticides are involved: clothianidin, imidacloprid and thiamethoxam. They may have acute and chronic effects on survival and development of bee colonies, their behavior and larvae.
Following this, the European Food Safety Authority (EFSA) limited the use of thiamethoxam, clothianidin and imidacloprid. Some European countries have banned or restricted the use of neonicotinoids.
 Table 1: List of commercial neonicotinoids
Table 1: List of commercial neonicotinoids
In order to better understand the effect of these compounds on bees and their contamination in pollen and honey, a highly sensitive assay method was necessary. For this purpose, an UHPLC-MS/MS analysis was developed including the controversial and well-known fipronil compound. Fipronil is a broad-spectrum insecticide of the phenylpyrazole chemical family and disrupts the insect central nervous system in the same manner as other neonicotinoids.
Materials and Methods
Standards and Reagents
All of the analytical standards were provided by Sigma-Aldrich. The Internal standards thiamethoxam-d3, imidacloprid-d4 and chlothinidin-d3 were purchased from Sigma-Aldrich. The solvents used, including water and mobile phase additive, were of UHPLC/ MS quality (Biosolve).
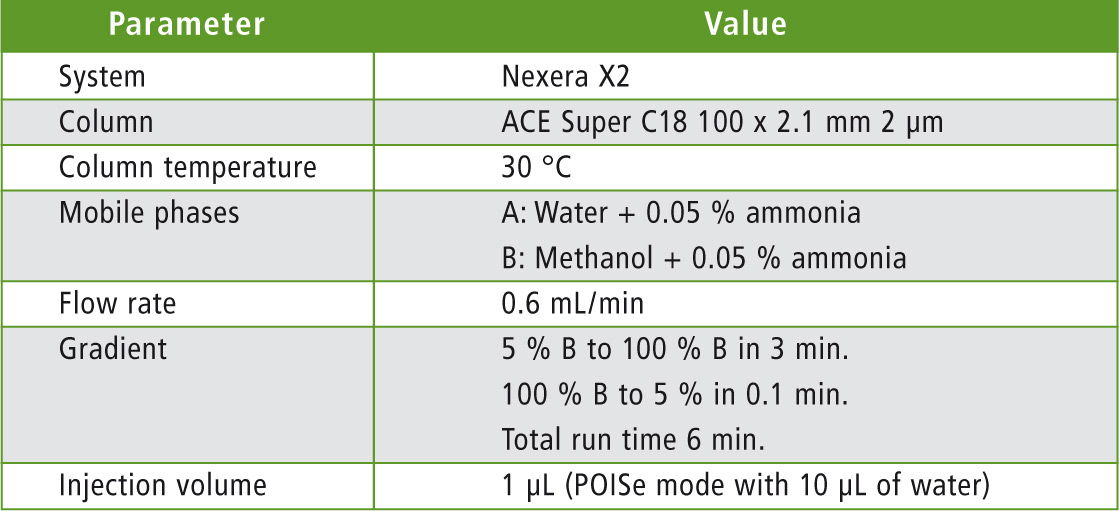 Table 2: UHPLC parameters
Table 2: UHPLC parameters
Sample Preparation
Compound extraction was performed using a QuEChERS method (Quick, Easy, Cheap, Effective, Rugged and Safe) with an additional dispersive Solid Phase Extraction (dSPE) step. 5 g of honey (±1 %) were weighed in a 50 mL polypropylene tube. 5 µL of internal standard solution at 5 µg/mL of each compound in acetonitrile was added to the honey and dried for ten minutes. 10 mL of ultra-pure water were subjoined, and the samples were homogenized by vortex mixing for one minute. 10 mL of acetonitrile were then added followed by vortex mixing for one minute.
Salts mix (4 g MgSO4, 1 g Sodium Citrate, 0.5 g Sodium Citrate sesquihydrate, 1 g NaCl; Biotage Q0020-15V) were added to the samples. After manual shaking, samples were centrifuged at 3,000 g for five minutes at 10 °C.
The supernatant (6 mL) was transferred into a 15 mL tube containing 1,200 mg MgSO4, 400 mg PSA and 400 mg C18 (Biotage Q0050-15V). After centrifuging at 3,000 g and 10 ºC for five minutes, the supernatant was transferred into inert glass vial for analysis (Shimadzu LabTotal 227-34001-01).
UHPLC-MS/MS Conditions
Analysis was performed using a Nexera X2 UHPLC system coupled with LCMS-8060 triple quadrupole mass spectrometer with Heated ESI in positive and negative ionization (figure 1). Mobile phase composition was optimized to generate the highest sensitivity. Ion source parameters (gas flows, temperatures) were also optimized using the Interface Setting Support Software (Shimadzu Corp.)
 Figure 1: Overview of the UHPLC-MS/MS system
Figure 1: Overview of the UHPLC-MS/MS system
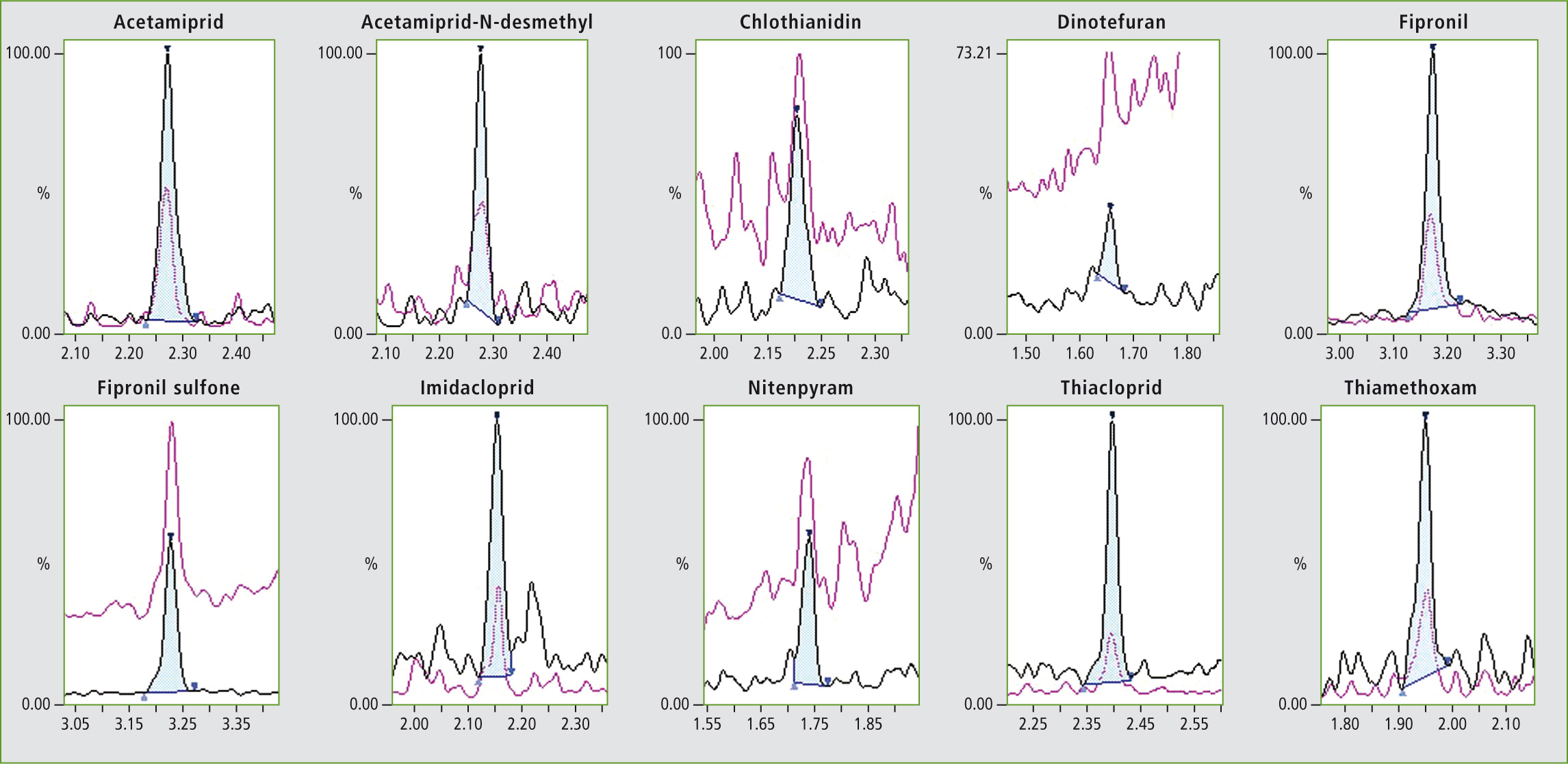 Figure 2: Chromatogram of the target compounds at their lower limit of quantification
Figure 2: Chromatogram of the target compounds at their lower limit of quantification
Results
Calibration
The calibration curves were prepared in acetonitrile in order to obtain final concentrations ranging from 2.5 pg/mL (2.5 fg on column) to 5 ng/mL. These concentrations correspond to 5 ppt and 10 ppb in honey respectively. A typical calibration curve is shown in figure 3.
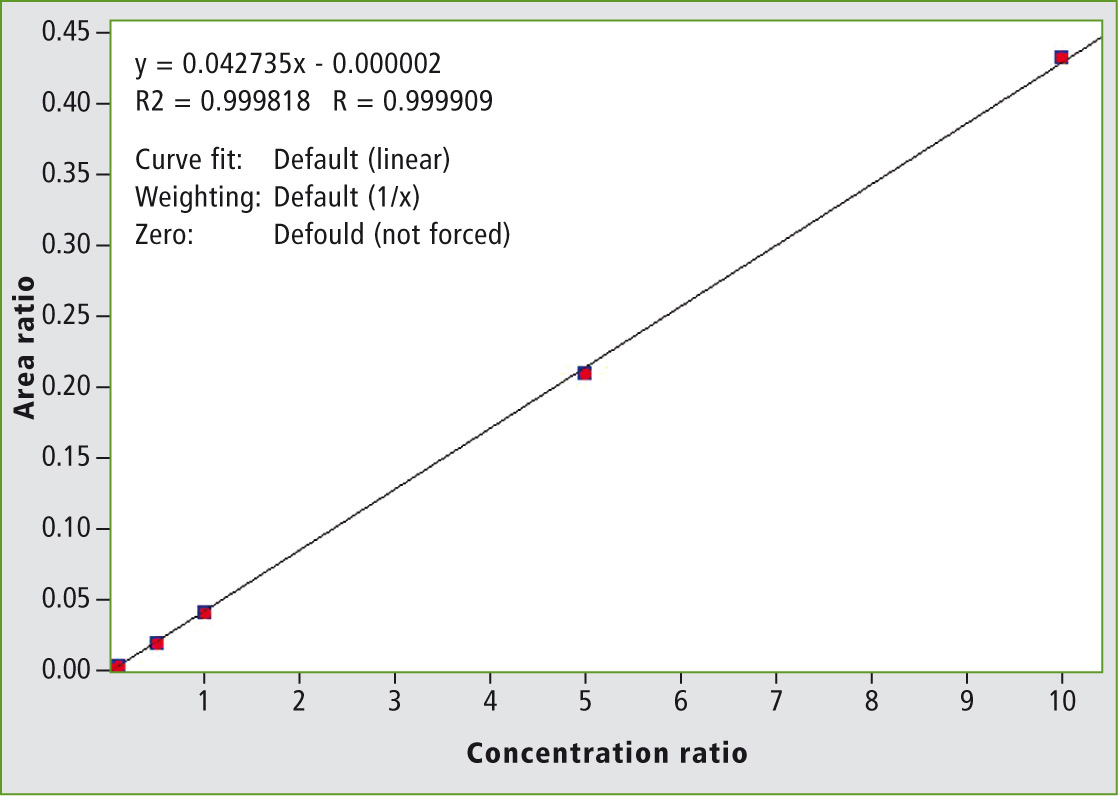 Figure 3: Calibration curve of clothianidin
Figure 3: Calibration curve of clothianidin
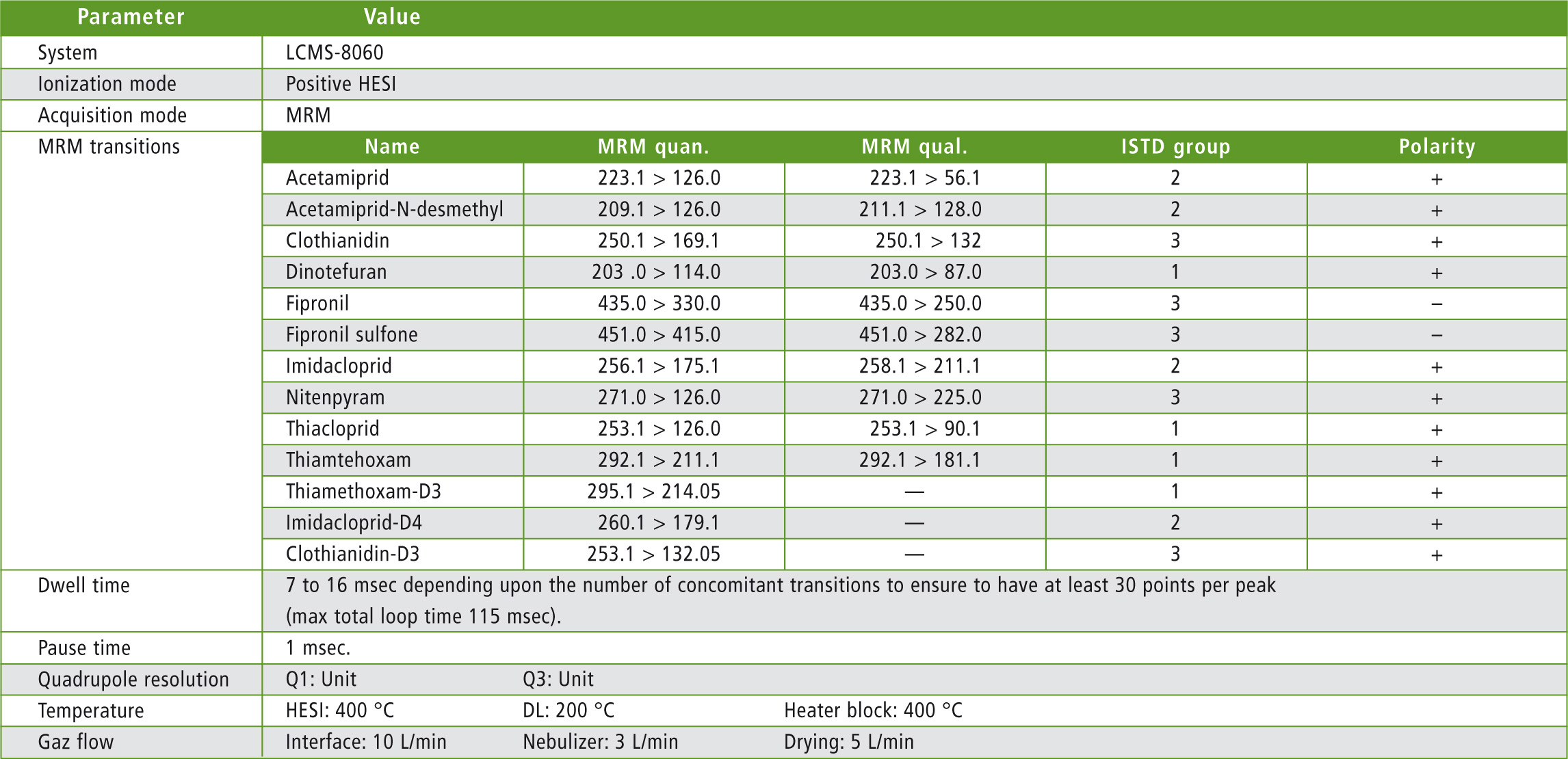 Table 3: MS parameters
Table 3: MS parameters
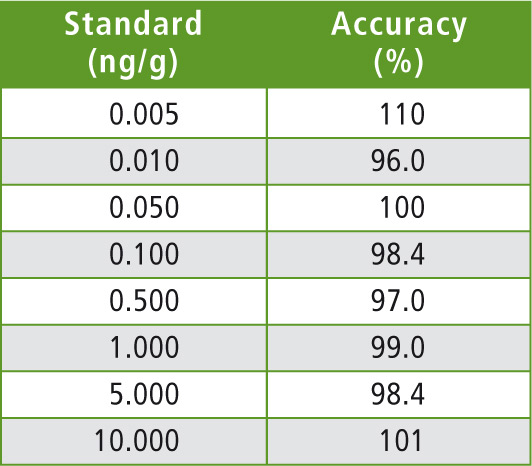 Table 4: Concentration for calibration and accuracy
Table 4: Concentration for calibration and accuracy
Recovery
An “all-flowers” honey from the local supermarket was extracted with or without spike at 50 ppt. A blank extract (no honey) was prepared to evaluate losses or non-specific interactions. Results are presented in table 5.
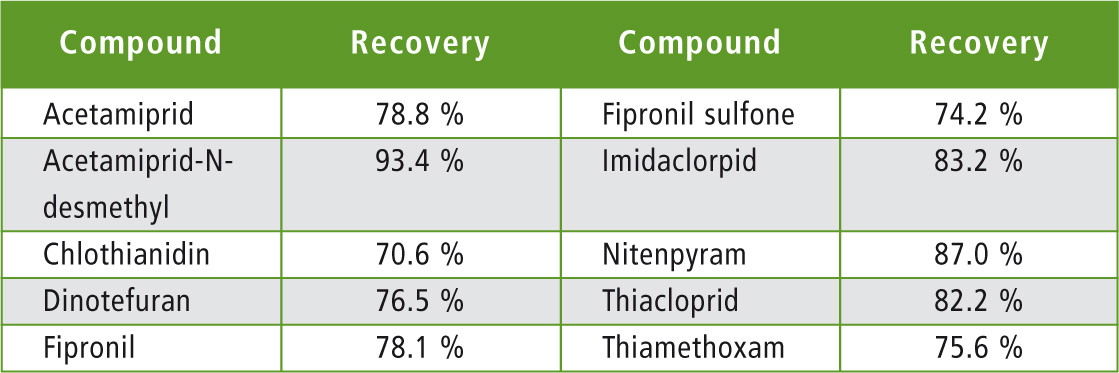 Table 5: Measured recoveries in honey
Table 5: Measured recoveries in honey
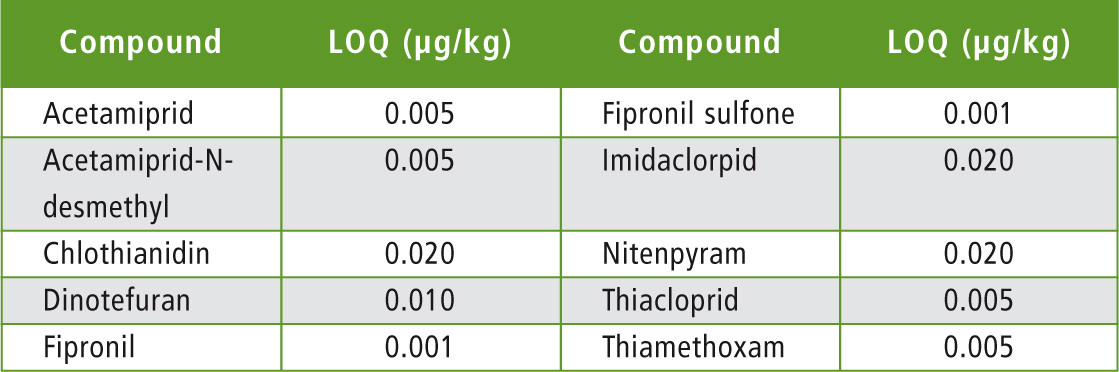 Table 6: Limits of quantification in honey
Table 6: Limits of quantification in honey
All calculated recoveries were within acceptance values of 70 – 120 % from EU SANTE/11945/ 2015.
Real Samples Analysis
Nine honey samples purchased at the local supermarket or used as raw materials in cosmetics (orange tree honey) were assayed as unknowns. All honeys tested showed concentrations far below the maximum allowable residue limit. But thanks to the very high sensitivity reached, even low concentrations of neonicotinoids were quantified. Results are presented in table 7.
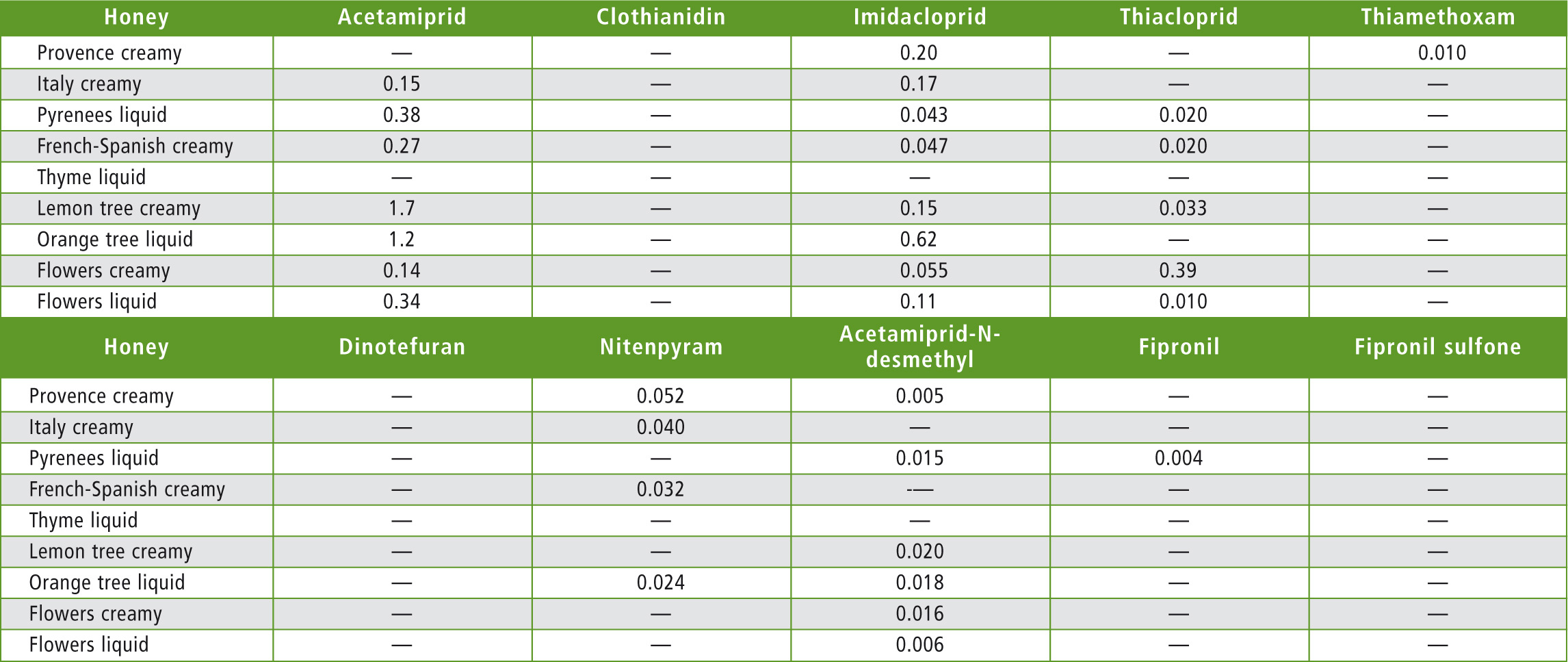 Table 7: Honey samples results (concentrations in µg/kg)
Table 7: Honey samples results (concentrations in µg/kg)
Stability
The thyme honey sample with no detectable target compound was spiked at 50 ng/kg with all compounds prior to extraction. The extract obtained was then consecutively injected 150 times in the system.
The results presented in figure 5 show excellent stability of the signal even at these low concentrations. This demonstrates that excellent sensitivity can be maintained over a long series of real sample analysis thanks to the ion source ruggedness.
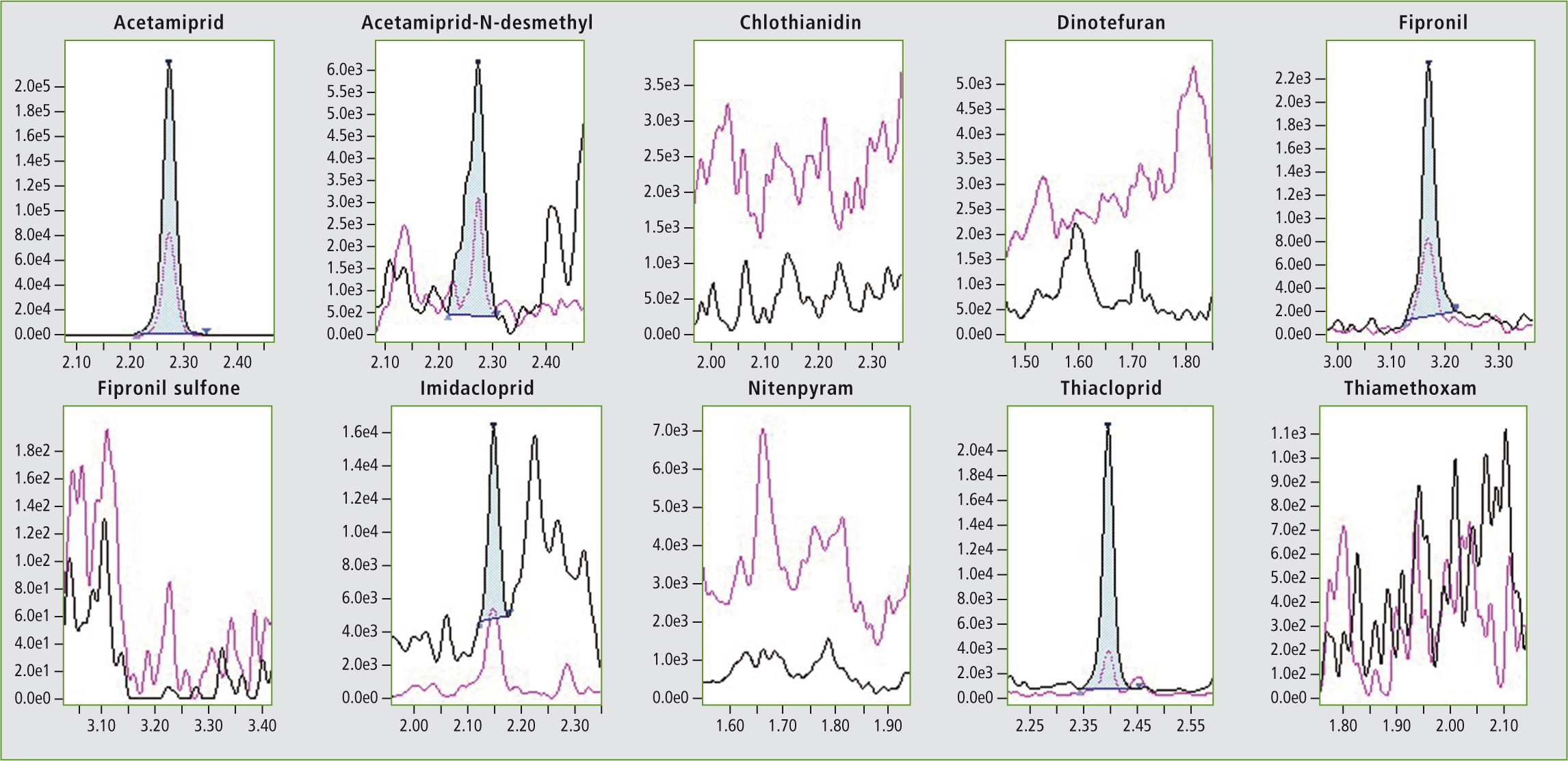 Figure 4: Chromatogram of a sample honey (pyrenees)
Figure 4: Chromatogram of a sample honey (pyrenees)
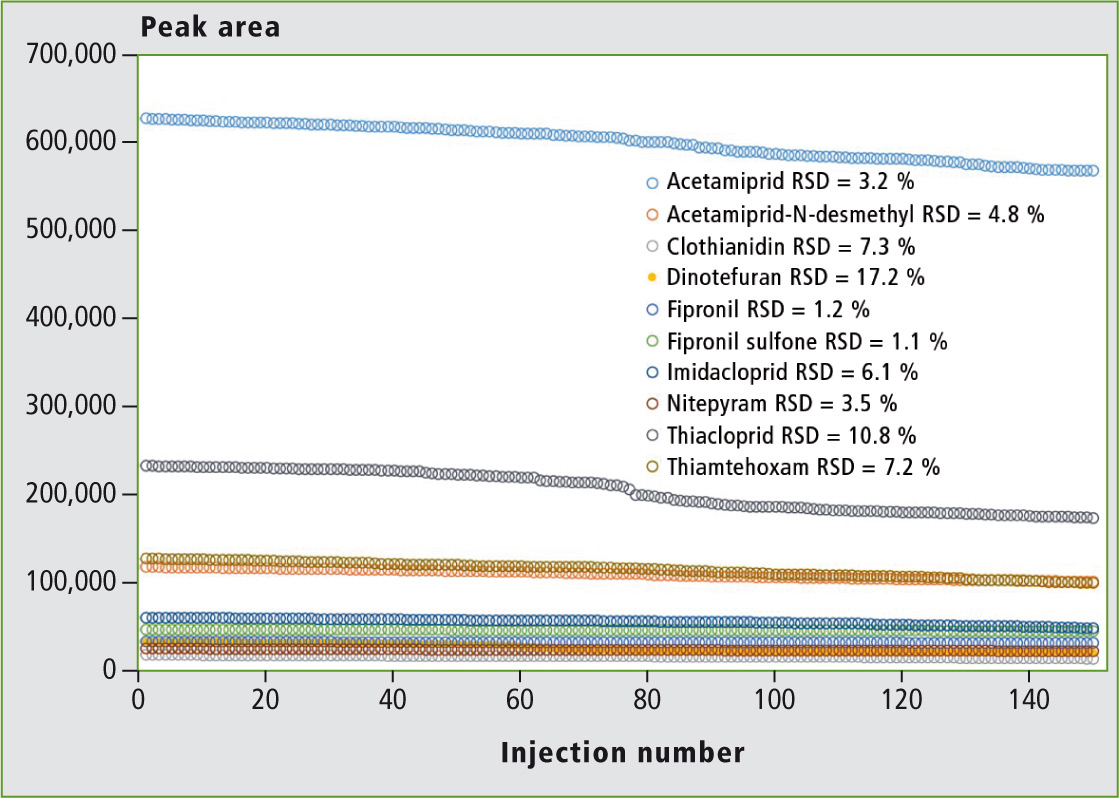 Figure 5: Stability of peak areas in real honey samples
Figure 5: Stability of peak areas in real honey samples
Conclusion
A method for ultra-sensitive assay, covering most neonicotinoids of interest including fipronil in honey was set up. Sample preparation was simple but provided excellent recoveries, whatever the honey type. The injection mode used prevented the use of tedious evaporation/reconstitution or dilution steps.
The sensitivity obtained enabled assay in real samples at very low levels far below the regulated residue levels. This method can be a very efficient support tool for better understanding of the impact of neonicotinoids on honey bee colonies.
Literature
Application note C140: Ultra-Sensitive and Rapid Assay of Neonicotinoids, Fipronil and Some Metabolites in Honey by UHPLC-MS/MS [LCMS-8060]
EU SANTE/11945/2015: Guidance document on analytical quality control and method validation procedures for pesticide residue analysis in food and feed.