From powder to pill – quality control of pharmaceutical goods
Active agent analysis using FTIR spectroscopy
In analytical instrumentation, FTIR measurement is the only technique providing non-destructive analysis of substances. It identifies powder, solid or liquid samples within shortest time, needing just milligrams of materials.
FTIR spectroscopy’s advantage is the ability to verify substances within 1 minute. Proof of identity is guided by documents prepared by the pharmaceutical industry as well as the national or European Pharmacopoeia. The European Pharmacopoeia set up a range of comments for each of Pharmacopoeia’s relevant substances. The same set of rules helps in its physical observations in qualification of the instruments. Some of the pharmaceuticals prepared for the export business are regulated by USP or FDA rules. The task of a modern FTIR is to be equipped with a validation concept which accounts for all cases.
More and more, a concept of easy-to-use and result-oriented analysis is required. A selection of functions is therefore needed which will select the requested comparison analysis based on Pharmacopoeia. A comparison can be:
- a simple visual comparison
- search in a library
- purity check
- comparison of dedicated signals
- contaminant analysis.
The cycle of measurement
The highly accurate measurement technique should still be fast. FTIR analysis with non-destructive approach supports these important aspects. A cycle of measurement can be described easily:
- take the sample
- place it in the measurement window
- press the sample (solids only)
- measure
- remove and clean the instrument’s window.
A time frame of one minute is a rough approximation for this handling cycle. Such simple and quick solutions must be supported by accessories of the same class, such as single reflection accessories enabling a simple quality control from raw material up to the final product, e.g. a tablet. In case of very hard substances, a diamond-based version of single reflection supports the application, pressing the substance onto a measurement window. A soft sample gives easy access to the IR spectrum because of a good contact between sample and window. In case of hard materials, more force is needed to establish a good contact.
The measurement technique is ATR (attenuated horizontal reflection). Depending on the optical elements used the analysis beam will penetrate the sample surface by approximately 2 µm. In the case of ready-to-use pills, the surface cover can be hard and blocks the pharmaceutical agent below. Such pills need only a cut. Capsules have to be opened, whereas film-styled surfaces can be cut. For example, capsules made for liquids can be cut and the liquid transferred onto the measurement window.
Active agent analysis with FTIR
A different aspect in pharmaceutical analysis is the packaging of the goods. It can be the pill capsule made from gelatin, sugar, and starch or it can be the blister carrying the pills and providing easy handling for the end-user. These blisters, metal foils as well as metal and plastic tubes for paste and creams can be analyzed with FTIR.
One sample of analysis will be presented here: the control of Metoprolol succinate, an active agent reducing blood pressure. Identification was carried out using FTIR-Spectroscopy.
Next to the active agent, other substances are contained in a tablet (Figure 1), determining color or contributing to stability. These fillers and accompanying materials including dye enable a regulated reception of the active agent (dissolution) by the human organism.
 Figure 1/2: A tablet with beta blocker based on Metoprolol succinate with 47.5 mg active agent out of 447.5 mg total weight / Structure of Metropolol
Figure 1/2: A tablet with beta blocker based on Metoprolol succinate with 47.5 mg active agent out of 447.5 mg total weight / Structure of Metropolol
High blood pressure is a creeping illness that can occur slowly over the years. It can cause heart disease, circulation problems and strokes. Pharmaceutical active agents based on Metoprolol represent an effective means to reduce high blood pressure. Common variants are Metoprolol tartrate and Metoprolol succinate. Tartrate and succinate are salts belonging to the class of bicarbonic acids, derived respectively from wine acid and amber acid. In the Pharmacopoeia, both materials are contained.
Composition declared
Active agent: Metoprolol succinate (PH. Eur) of beta blocker 47.5 mg per tablet
Contents: Cellulose (E460), Polyvinyl pyrrolidone, glucose, Lactose-Monohydrate, Macrogol 4000 (Polyethylenglycol), magnesium stearate (PH. Eur), corn starch, Polyacrylate, Silicon dioxide, Sucrose, talcum, titanium dioxide as a dye (E171)
The active agent Metroprolol is described as follows: (Metoprolol) 2 ‘HO2C-CH2-CH2-CO2H, sum formula C34H56N2O10; molecular weight of the racemic mixture Mr = 653 g/mol. The active agent makes up approx. 10 % of the total weight of the tablet, which should be detectable in the complex mixture.
Measurement
- The tablet was first tested in its as-is state.
- A single-reflection-ATR-unit was used.
- The IR-spectrum of the surfaces measured determined starch and other filling materials (Figure 2).
The tablet was then divided in two, and a cross-section was taken. This powder granular material was then measured (Figure 2, red spectrum).
When comparing the spectra, clear differences are apparent. The surface spectrum (penetration depth of the light beam approx. 2 µm) of the filler material highlights a strong signal around 1,100 cm-1, covering many of the components in the contents list. Starch, cellulose and sugar (in the form of Lactose and Sucrose) influence this signal.
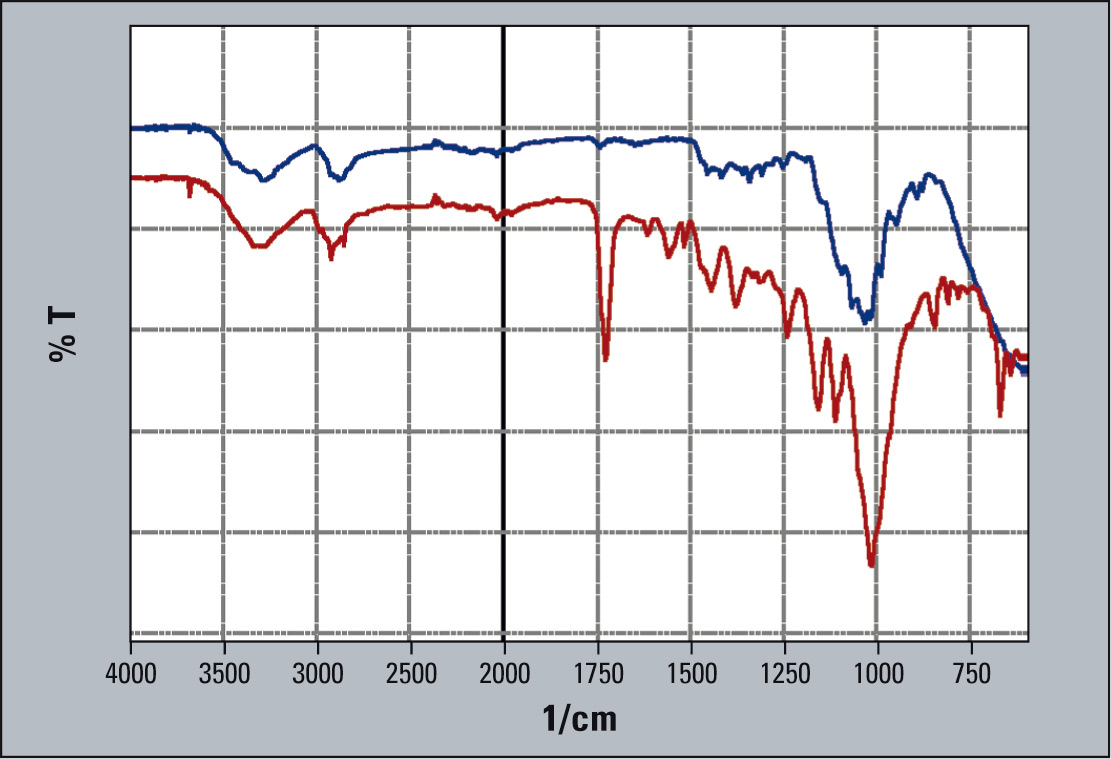 Figure 2: IR-spectrum of the surface measurement of the tablet using a single reflection unit (blue) and IR-reflection-spectrum of aparticle from the inside of the tablet (red)
Figure 2: IR-spectrum of the surface measurement of the tablet using a single reflection unit (blue) and IR-reflection-spectrum of aparticle from the inside of the tablet (red)
When observing the second spectrum in figure 2, filler materials with their infrared signals are present, but the spectrum is located around some dissolved signals. Interestingly, a secondary Amino molecular group appears which is from the active agent Metoprolol; as do oxygen atoms in the form of Ethoxy-, Phenoxy- or Hydroxy-groups, as well as a 6-atom ring molecule.
For filler materials, interpretation of the signal in the IR-spectrum is more complex than with analysis of the pure materials. However, some significant signal vibrations can be recognized unambiguously: Metroprolol and Succinate (Table 1). The vibrations representing Succinate could not be found in the spectra of the filler materials or in the spectra of the pure materials of Metoprolol and Metoprolol tartrate (Sadtler Crime/Forensic Libraries).
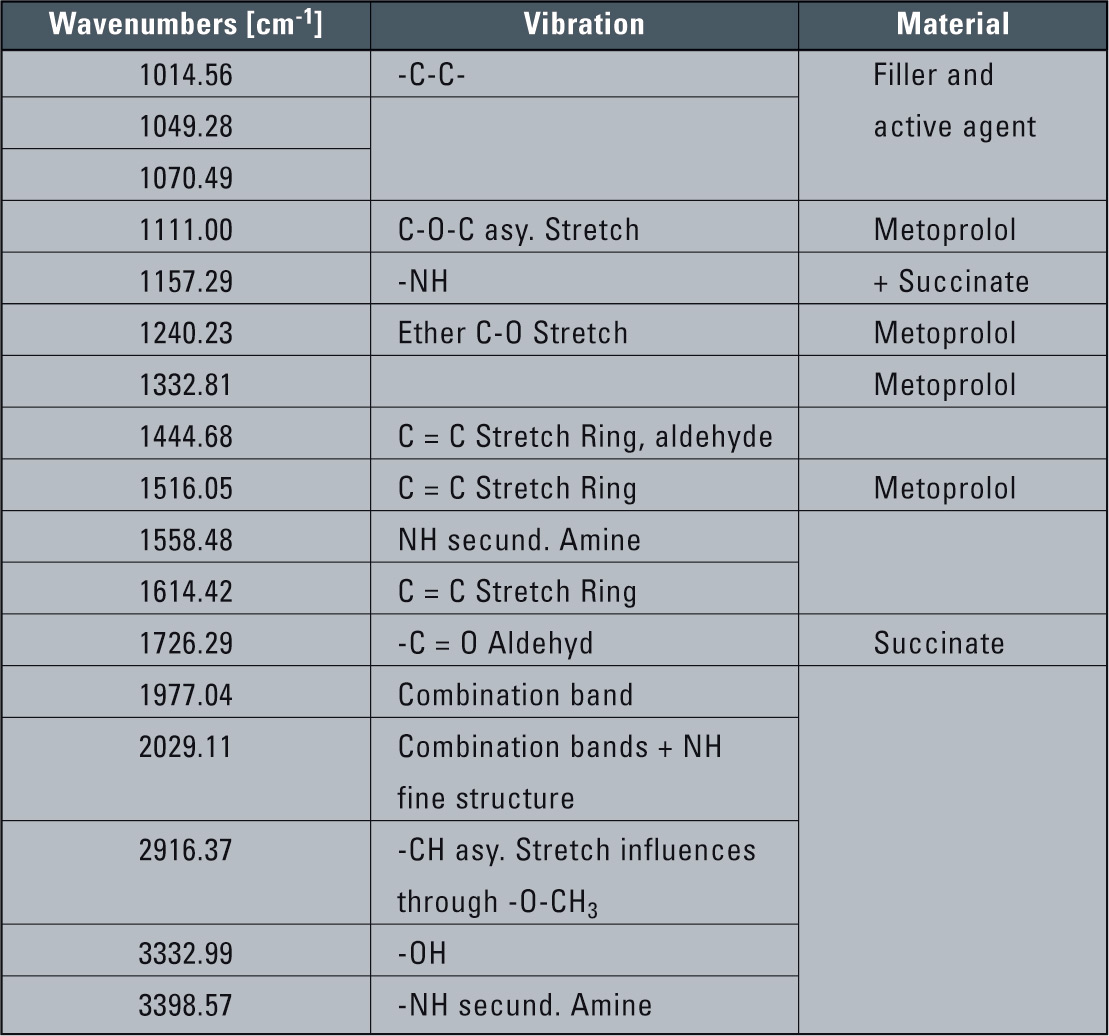 Table 1: Coordination of the vibration signals
Table 1: Coordination of the vibration signals
An intensive CO-signal volume is noticeable in 1,726 cm-1, that could be identified as Succinate. Furthermore, a signal doublet was found similar to Succinate in 1,111 and 1,157 cm-1 with a difference of 46 cm-1, corresponding to a doublet of the amber acid (1,084 and 1,132, with a difference of 48 cm-1). The signal deviation can be explained by the different molecule interactions in the salt in comparison to the acid (approx. 20 cm-1).
IR-spectra of tablets with 47.5 mg and 95 mg are shown in figure 3. The active agent’s increase in concentration can be recognized by a reduction in the single signal heights.
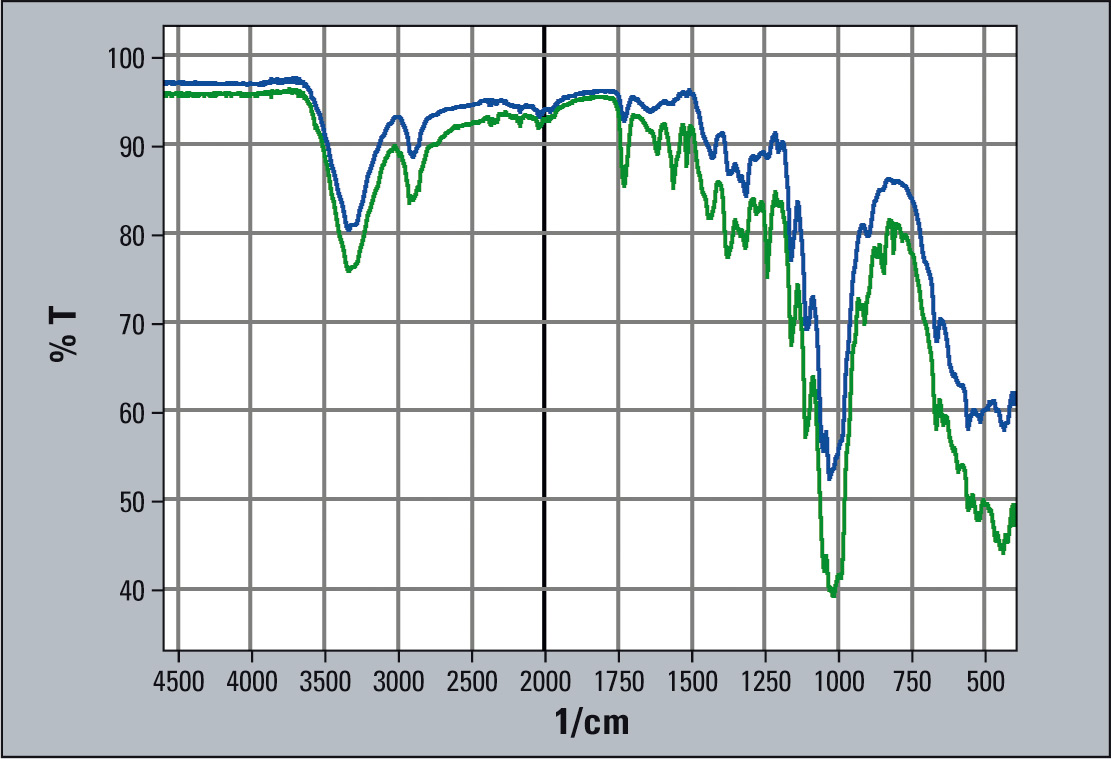 Figure 3: Infrared spectra of tablets with low and high dosage agent (blue = low, green = high dosage)
Figure 3: Infrared spectra of tablets with low and high dosage agent (blue = low, green = high dosage)
Instruments
FTIR-instrument: IRprestige-21
Software: IRsolution including search function
Accessories: DuraSamplIRII
Libraries: Shimadzu Pharmaceutical, Sadtler
Accessory
A single-reflection-ATR-unit was used. The measurement was carried out by simple dropping and pressing of the sample.