Better Prep!
New Nexera Prep LC for a simplified and highly efficient preparative workflow
The new Nexera Prep series of preparative and purification LC systems improves productivity tremendously via efficient preparative work using application-specific LC or LC-MS methodology. It offers ease-of-operation in a flexible set-up and enables customer- and application-specific solutions, supported by a wide choice of equipment, accessories, detectors and efficient process automation. Nexera Prep provides better prep processes for drug discovery and purification of functional components in pharmaceutical, chemical and food industries.
 Nexera preparative LC system
Nexera preparative LC system
The typical preparative workflow follows several steps that can be streamlined using the latest technology:
1. Development and optimization of separation conditions in analytical scale
In order to isolate different analytes from a mixture of components, separation of the compounds of interest must be ensured. Analysis and fractionation parameters have to be carefully optimized. Since method development in preparative scale would waste a lot of sample and eluent, the analytical scale Method Scouting system for automated column screening with a variety of mobile phases is the ideal tool for this purpose.
2. Upscaling from analytical to preparative LC conditions
After optimization of separation conditions in analytical scale, upscaling from analytical to preparative LC conditions can be calculated using the dedicated “Method Transfer” tool. The dimensions and particle size of the preparative separation column should be selected according to the required amount of purified compound or injection volume as shown in table 1. Column dimensions dictate the flowrate range and the appropriate system configuration can be determined based on mobile phase flow required, target number of fractions and fraction volume.
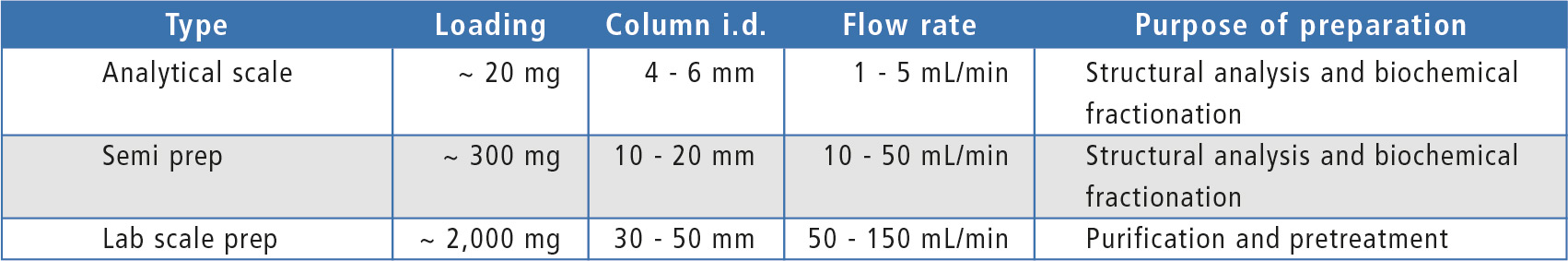 Table 1: Typical loading, column dimensions and flow rate in different scale LC analysis
Table 1: Typical loading, column dimensions and flow rate in different scale LC analysis
3. Optimization of fraction conditions for preparative work
LabSolutions software offers precise fractionation simulation, where a peak segment in the pre-preparative results can be specified, and fractionation parameters are set automatically in the system, thereby reducing time and effort significantly. Signals from up to four channels of a variety of detectors enable most selective and sensitive identification of target compounds.
4. Re-analysis of collected compounds to confirm purity
With the new LH-40 Liquid Handler for re-injection of isolated fractions, purity of target compounds can be confirmed using a dedicated analytical flow path of the same versatile system.
Senna powder: Nexera Prep in an application example
Preparative purification of sennoside A and sennoside B from senna powder and confirmation of purity in a single system.
Sennoside A and sennoside B are the active components in senna leaf extract powder, commonly used as a natural laxative. In order to isolate the target compounds, a HPLC separation was developed in analytical scale and transferred to semi-preparative conditions. After successful fractionation of the single analytes, the samples collected were subjected to re-analysis to confirm the purity of each compound.
Figure 1 shows typical chromatograms of a) 50 µg/mL mixed standard solution of sennosides A and B and b) senna powder extract in methanol. For preparation of the extract sample, 100 mg senna powder was extracted for 30 min in an ultrasonic bath with 10 mL of a 70 % aqueous solution of methanol, centrifuged and the supernatant was filtered before HPLC analysis. Because of the large amount of contaminating components in the extracted powder sample, the column had to be rinsed thoroughly with 100 % organic solvent after elution of the separated target components.
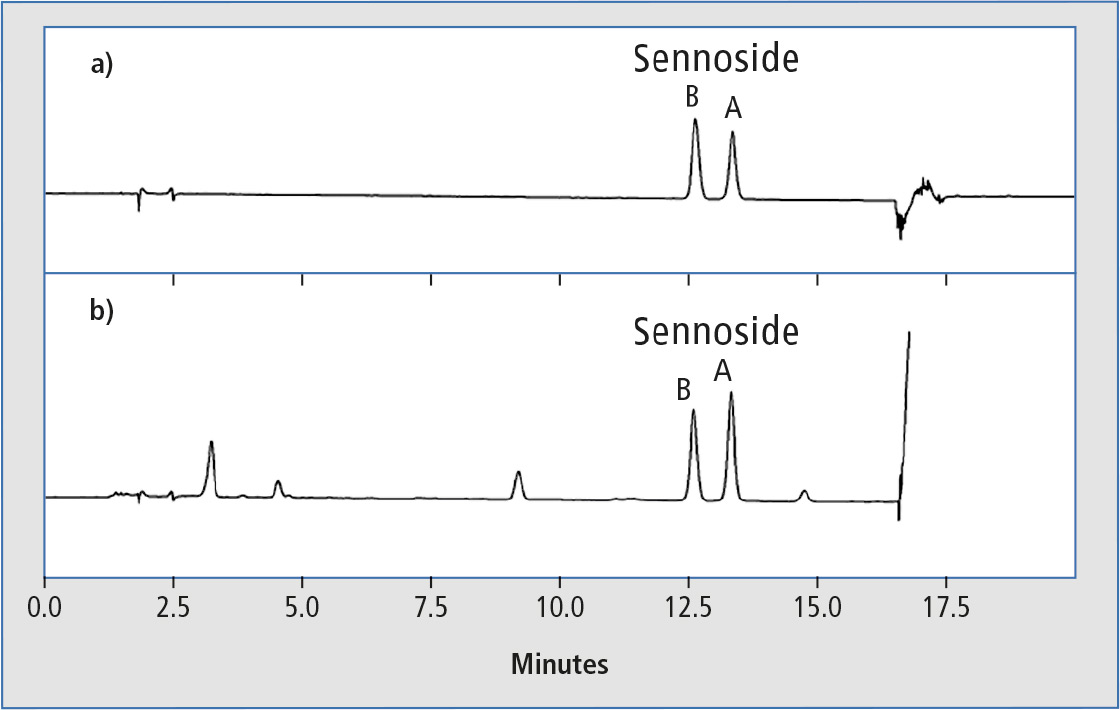 Figure 1: Typical chromatograms of a) 50 µg/mL mix standard solution of sennosides A and B and b) senna powder extract in methanol
Figure 1: Typical chromatograms of a) 50 µg/mL mix standard solution of sennosides A and B and b) senna powder extract in methanol
Analytical separation conditions
Column: Shim-pack Scepter C18, 150 x 4.6 mm, 5 µm
Mobile phase A: 0.1 mol/L ammonium acetate in water (pH 6.9)
Mobile phase B: Acetonitrile
Gradient: 5 % B → 12 % B (0 – 15 min)
12 % B → 100 % B (15.01 – 20 min)
100 % B → 5 % B (20.01 – 30 min)
Flow rate: 1 mL/min
Temperature: Ambient
Detection: UV 340 nm
Inj.Vol.: 2 µL
The LabSolutions method transfer calculator and fractionation simulator were used to convert the method to preparative scale and to specify the peak segments, for the scan system to automatically determine the appropriate parameters required for successful target fractionation.
Preparative separation conditions
Column: Shim-pack Scepter C18, 150 x 20 mm, 5 µm
Mobile phase A: 0.1 mol/L ammonium acetate in water (pH 6.9)
Mobile phase B: Acetonitrile
Gradient: 5 % B → 12 % B (0 – 15 min)
12 % B → 100 % B (15.01 – 20 min)
100 % B → 5 % B (20.01 – 30 min)
Flow rate: 19 mL/min
Temperature: Ambient
Detection: UV 340 nm
Inj.Vol.: 500 µL
Fractionation: 13.09 – 13.63 min (Sennoside B)
13.88 – 14.48 min (Sennoside A)
A typical chromatogram of the separation of sennosides A and B in preparative scale showing the color-coded fractionation segments can be seen in figure 2.
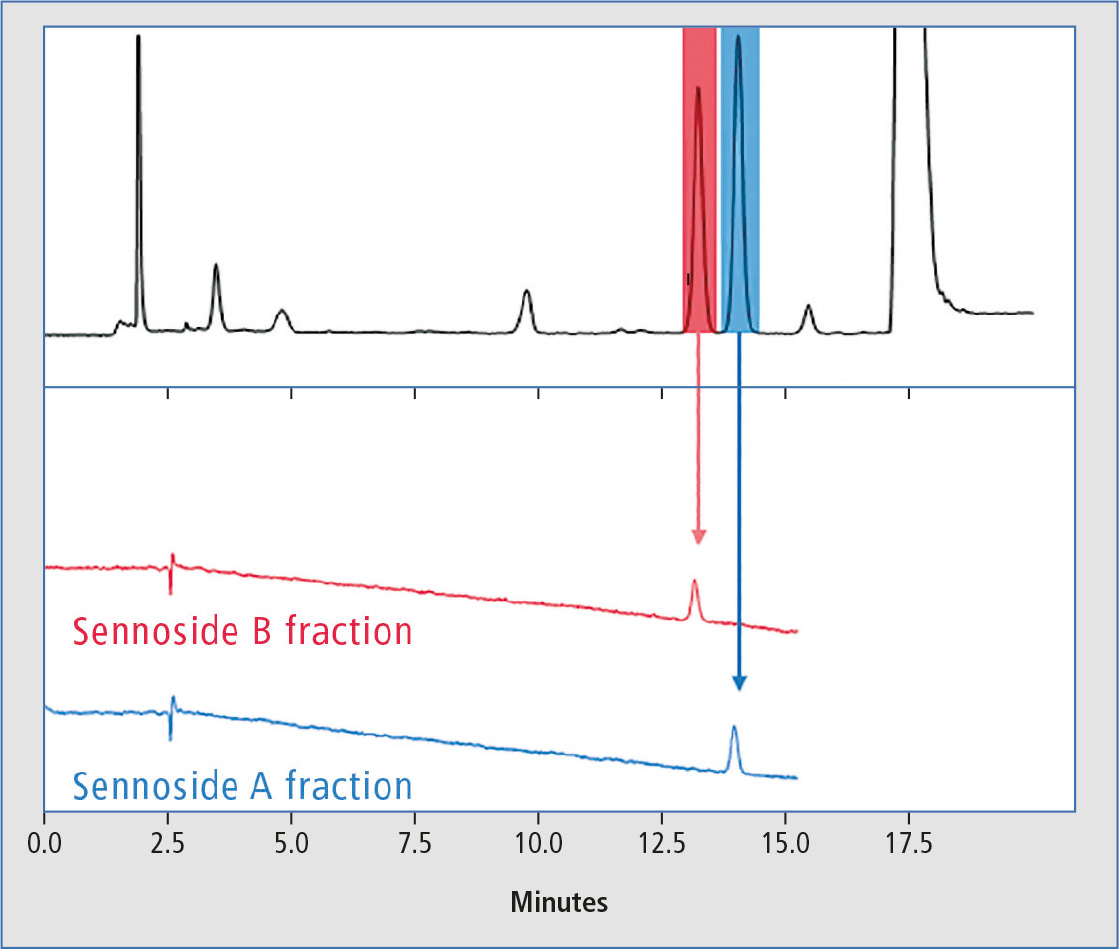 Figure 2: Typical chromatograms of the separation of sennosides A and B in preparative scale and re-analysis of single fractions
Figure 2: Typical chromatograms of the separation of sennosides A and B in preparative scale and re-analysis of single fractions
Conclusion
The Nexera Prep system for fractionation and purity confirmation was applied successfully to separate and isolate sennosides A and B from a mixed extract of senna powder. The system was equipped with the LH-40 Liquid Handler, to re-inject the collected fractions in a separate analytical flow path in the same system for confirmation of purity. The chromatograms obtained from re-analysis in analytical scale only showed the single peak for sennoside A and sennoside B respectively (figure 2).
