Implant contaminated?
TOC analysis in the production of orthopedic implants
Mankind explores the vastness of the cosmos and sends telescopes into space. Humans also explore the microcosm; with a particle accelerator, they break down matter into its components in order to uncover the big bang of the universe. 50 years ago, the first astronaut set foot on the moon, brought there by a lunar module with new electronic control technology. Hardly any comparison to today’s powerful devices, which allow access to enormous knowledge at the touch of a finger and connect people all over the world. These are amazing masterpieces of human ingenuity.
But often, the human body, one of the greatest miracles, falls out of sight. From a single cell an organism grows which has still not been fully understood in its entirety, although it has hardly changed over recent generations. And yet, life expectancy is increasing constantly due to better living conditions such as nutrition, hygiene, social stability and especially due to advances in medicine, such as diagnostics, drugs and medical devices.
Balancing benefits and risk
Medical devices today are key to support or even enable the recovery of patients from a variety of ailments, from bone fractures and joint damage to heart diseases and spinal injuries.
However, bringing foreign substances as implants into the organism is not possible without considerable potential risks for the patient – post-operative infections or long-term damage caused by heavy metal poisoning would be a catastrophe. It must therefore be ensured that so-called “biocompatibility” is always achieved, so that the benefit for the patient outweighs the potential risks. In the distribution and production of medical devices, manufacturers are therefore confronted with consistently strict quality requirements under regulatory supervision.
The new EU Medical Device Regulation MDR 2017/745
The European Parliament has passed the new medical device regulation MDR 2017/745 and transitional periods for product certification will already expire for manufacturers in May 2020. It replaces the previous Medical Device Directive MDD
Risk reduction through implant purity
Besides the actual material, hygiene and purity are key factors in achieving the biocompatibility of an implant. The international standard ISO 19227 “Cleanliness of Orthopedic Implants – General Requirements” is a guideline to assist manufacturers in addressing this issue. It covers general requirements from risk assessment and validation of cleaning methods to sampling, and prescribes a series of tests to demonstrate cleanliness throughout the entire production process. These include test methods to identify inorganic and particulate contaminants, organic contaminants and systematic visual checks. The TOC parameter is one way of determining the total organic contamination.
Validation of purity by TOC analysis
TOC (Total Organic Carbon) analysis records the total carbon from organic compounds in one step and is therefore particularly suited to determining contamination by organic components. Carbon content of the sample is oxidized to CO2 and analyzed with a NDIR (non-dispersive IR) detector. The parameter is common practice in cleaning validation for pharmaceutical production plants.
TOC measurement does not identify contamination, but does reflect the total organic contamination caused by by-products of the manufacturing process such as grease or lubricants, detergents and disinfectants as well as natural organic matter (biological contaminants). Aqueous samples after extraction can thus be analyzed quickly and easily (analysis time: approx. 4 min). A prerequisite is good water solubility of the substances to be tested. With the TOC analyzer of the TOC-L series, Shimadzu offers a very suitable tool for validating the cleanliness of orthopedic implants after liquid extraction.
Examination of a knee implant
Using the femoral component of a knee prosthesis as shown in figure 1, organic impurities on the surface of the sample were examined. Two beakers, each containing 250 mL ultrapure water were prepared, the previously cleaned implant being placed in one for actual extraction and the other used for blank value determination.
Both containers were left in a tempered ultrasonic bath for one hour for extraction. The extraction solutions of both containers were then filled into autosampler vials and analyzed in the TOC-L.
 Figure 1: Knee implant
Figure 1: Knee implant
To simulate different types of contamination, the implant was placed in a glucose solution containing 50 mg/L TOC for one hour. In a second experiment, an employee briefly touched the cleaned part with unprotected fingers. Both times, extraction solutions were produced and analyzed according to the above scheme.
The results in table 1 show that the organic contamination on the surface of the implant could be detected and confirmed quickly and precisely. ISO 19227 recommends a TOC limit of 0.5 mg per implant if historical data is not available. However, this is only an orientation – the actual limits should always be determined after a risk assessment, taking into account factors such as the size of the component, detergents used and potential sources of hazard.
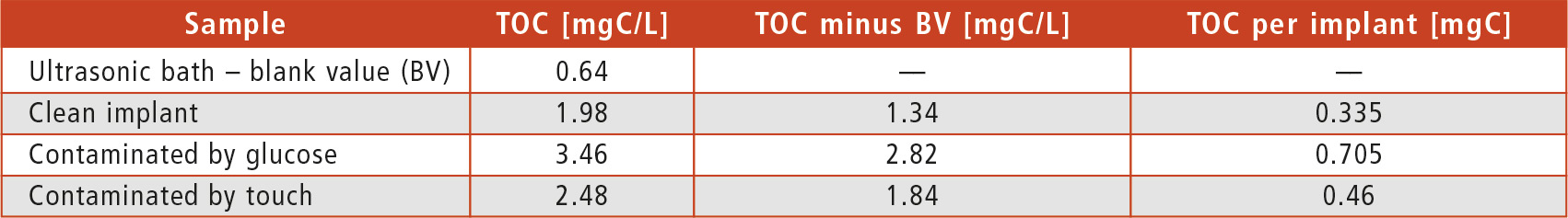 Table 1: Results of the extraction experiments
Table 1: Results of the extraction experiments
Alternatively, entire components such as smaller plates and screws for surgery as a whole could be examined for organic contamination in the TOC solid sampling module. In order to track down substances that are not water-soluble, analysis by means of GC-FID after extraction in non-polar solvents is a suitable method.
Summary
Shimadzu’s TOC-L series TOC analyzers are reliable companions in monitoring the production of implants and medical devices. Software packages and services additionally enable validated work within GxP-compliant data integrity. For many other test methods listed in ISO 19227, such as ICP-MS/OES, GC-FID, FTIR and particle determination, Shimadzu offers high-performance solutions.
Further information on this article
Application: »LAAN-A-TC-E049 074 – Cleanliness Evaluation of Orthopedic Implants using TOC«
Application: »LAAN-A-TC-E045 – Quality Evaluation of Medical Devices using TOC Solid
