Technical failure or ‘human factor’?
Measurement inaccuracies in X-ray fluorescence spectroscopy
In elemental analysis, ICP, AAS and EDX methods differ in terms of sample preparation complexity, lowest possible detectable concentrations, the number of simultaneously measurable elements and the overall measurability of an element. While ICP and AAS offer better detection limits, sample preparation can usually be omitted when using EDX. Carbon to uranium or sodium to uranium can be measured in solid as well as in liquid samples. Depending on the element and sample, the detection limit reaches down to the single digit ‘ppm range.’
 Figure 1: Energy dispersive X-ray fluorescence spectrometer EDX-800P
Figure 1: Energy dispersive X-ray fluorescence spectrometer EDX-800P
After selecting a suitable method for an analytical question, it is important to be able to determine the accuracy of the measurement results. Sometimes the analytical method or the measurement instrument are blamed for non-optimum measurement results or too high overall standard deviations.
The total measurement error, however, is the sum of many individual errors. The first error often arises during sampling. In this step, it is important to obtain a sample which is as representative as possible. Furthermore, the sample should be homogenous, and all elements present in the sample must be available for the applied measurement method. It can, however, still be possible that the sample itself changes the measurement result, either as a result of interaction between the sample components or due to interaction with the measurement method. Weighing errors or errors due to incorrect calibration distort the final result in addition.
The instrument error is often the smallest factor contributing to the total error of a measurement result.
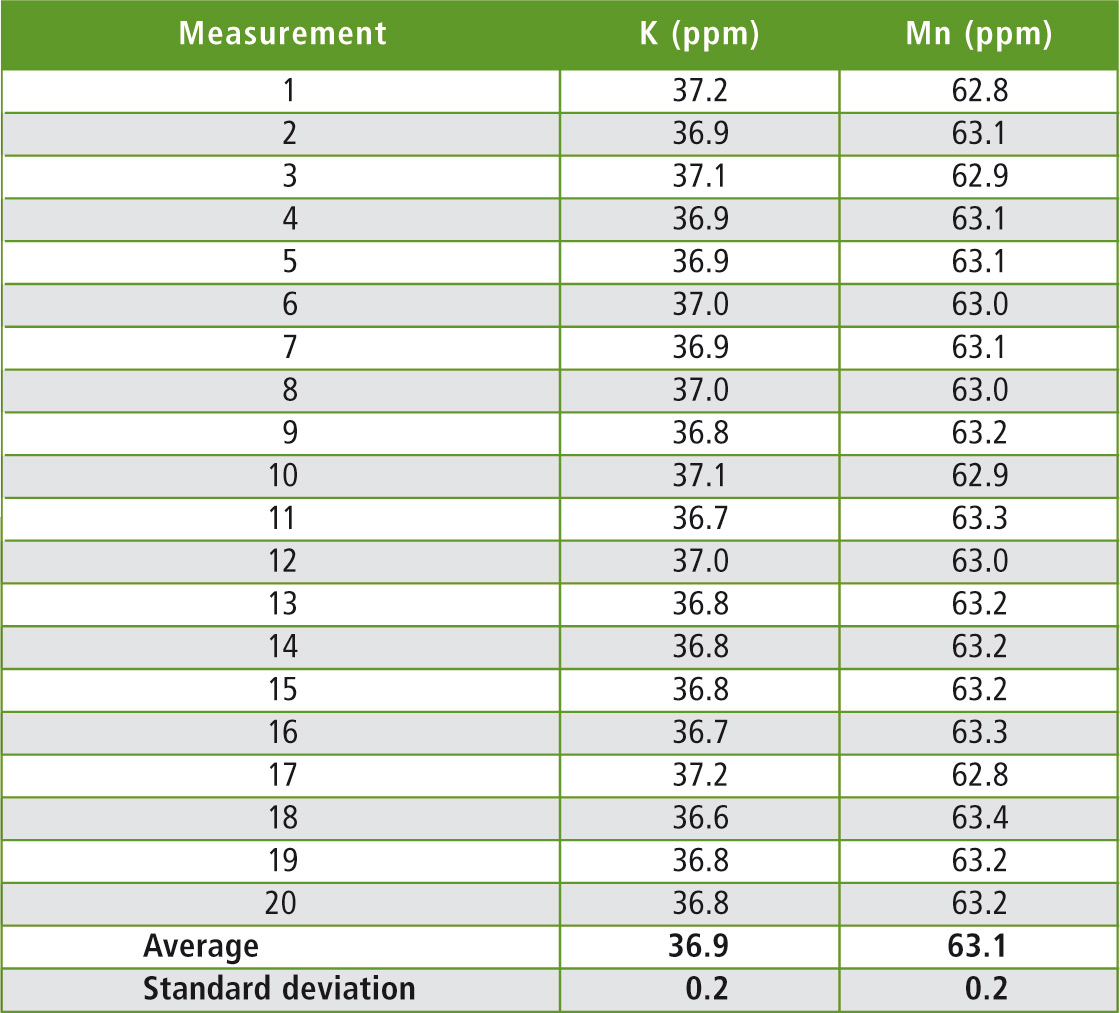 Table 1: Measurement results: K/Mn mixtures
Table 1: Measurement results: K/Mn mixtures
In the present case, a potassium/manganese mixture was finely ground, pressed into a tablet and measured 20 times using Shimadzu’s EDX-800P. The sample was not repositioned in the sample chamber, thereby excluding errors arising from possible sample inhomogeneity.
A standard deviation of the measurement results of 0.2 % was achieved. This reflects the measurement accuracy of the instrument in the present case.
Looking at the total error of an EDX measurement, 5-fold up to 10-fold higher standard deviations are quite realistic. It is a recurring challenge for the experimenter to reduce these errors as much as possible using suitable sampling and sample preparation methods. This is a challenge in which Shimadzu is happy to assist.