Validated method for monoclonal antibody drugs
Assessment of the nSMOL methodology in the validation of bevacizumab in human serum
Development and validation of bioanalytical methods for proteins can be hindered by the availability of assay reagents. LC-MS/MS hybrid assays enable an approach capable of using more generalized reagents. For these assays, the capture and subsequent sample processing steps are highly critical for optimal sensitivity and selectivity.
Nano-surface and molecular-orientation limited proteolysis (nSMOL) provides a unique sample processing approach to quantification of monoclonal antibody (IgGs) drugs in biological samples. The nSMOL technique provides selective proteolysis of the Fab region of the antibody using a specialized protein A capture resin and trypsin-immobilized nanoparticles for protein digestion.
Evaluation of the method with a cancer-treating medication
Shimadzu scientists have tested this technology and successfully developed and validated methods for several monoclonal antibody drugs [1, 2]. As part of an independent evaluation of the methodology, Intertek Pharmaceutical Services (a contract research organization based in San Diego, CA) has evaluated the technique and validated an LC-MS/MS method for bevacizumab using a validation protocol consistent with industry and regulatory standards for pharmaceutical drug candidates [3, 4]. Bevacizumab is a medication applied to treat a number of types of cancer.
To assess the utility of the nSMOL technique, an LC-MS assay was developed for the analysis of bevacizumab in human serum with a standard curve range of 100 ng/mL to 10,000 ng/mL. Initially, the standard Shimadzu sample processing protocol using the kit analogue internal standard was followed and yielded results with acceptable accuracy and precision. However, for the chromatography developed internally, the analogue internal standard gave atypical results at high analyte concentrations in frozen QC samples.
Applicable for the rapid development of methods with minimal assay optimization?
The method was subsequently optimized to a 96-well plate method with stable labeled peptide internal standard for improved throughput, selectivity and assay performance. No substantial changes were made to the critical Shimadzu protocol steps to test whether or not the methodology can be used to rapidly develop methods with minimal assay optimization. Using 10 µL of serum, a LLOQ (lower limit of quantification) with strong peak response (>20 : 1 S/N) was observed. Conversion of the assay to a 96-well format required a change in the aliquot size of the method due to an unknown drop in sensitivity, but 10 µL was deemed to be a more than acceptable assay volume.
Assay performance during the validation was acceptable and well below the ±20 % acceptance criteria (table 1) with inter-assay %Dev values ranging from -5.89 % to -1.66 % (2.17 % at the LLOQ) and inter-assay precision values at ≤ 7.05 % (13.8 % at the LLOQ). Analyte peak response in blanks of all individual human serum lots evaluated was <20 % of the LLOQ peak response, showing acceptable selectivity.
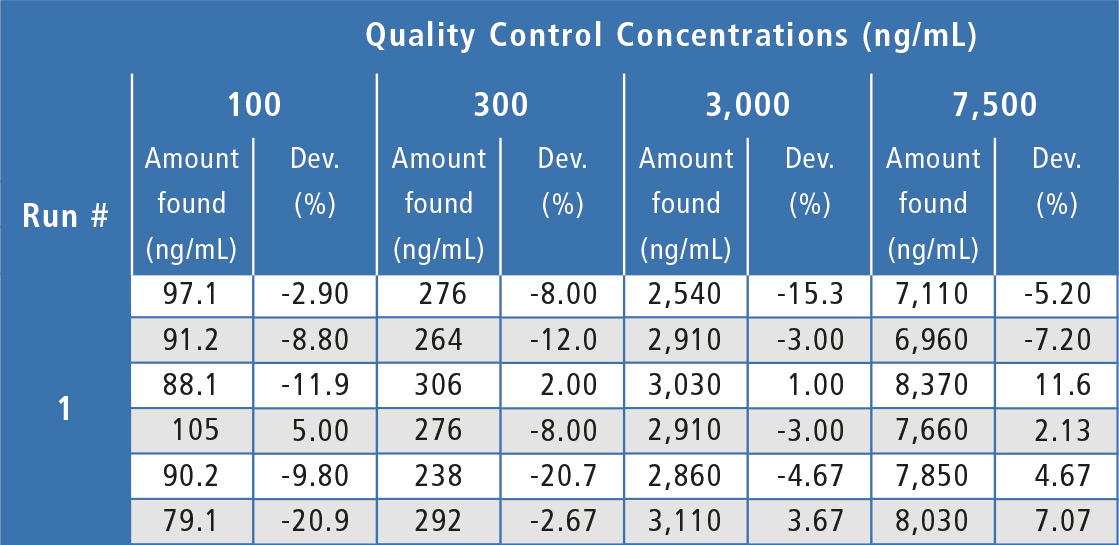
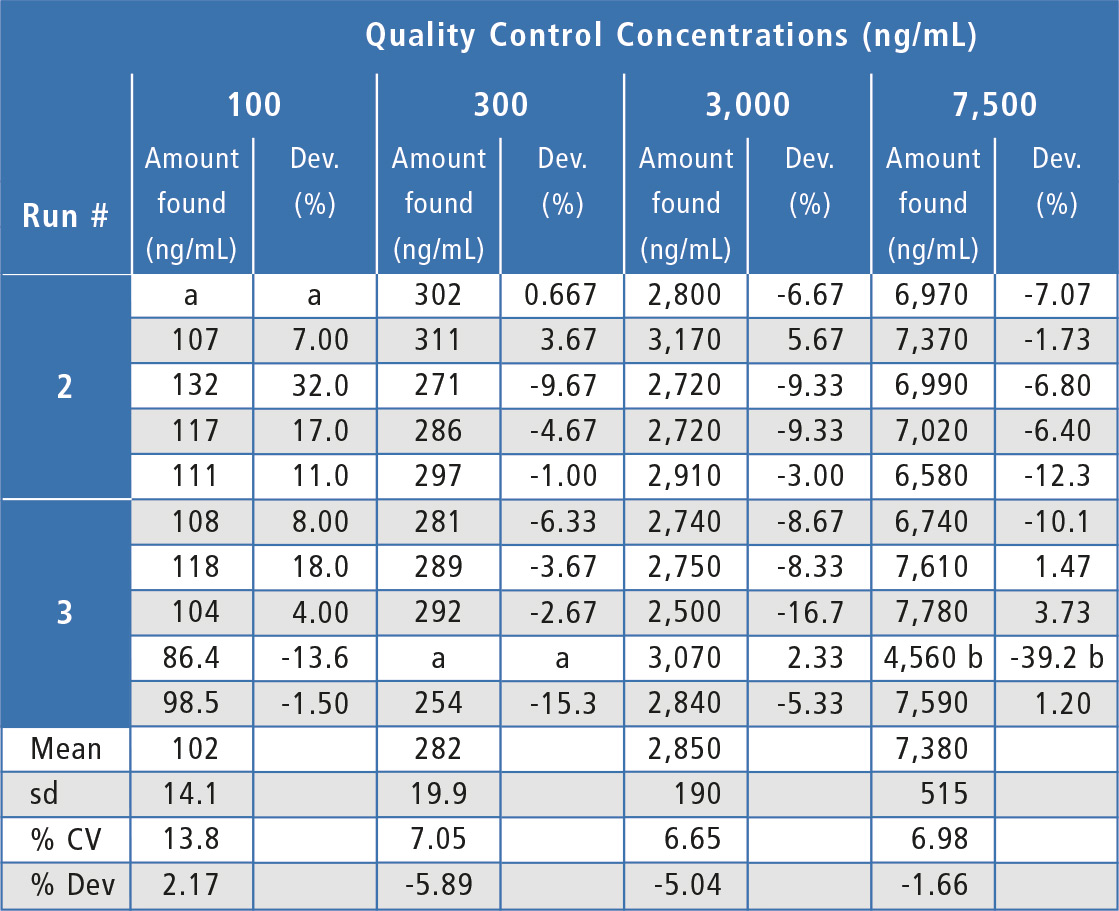 Table 1: Quality control results for Bevacizumab in human serum.
Table 1: Quality control results for Bevacizumab in human serum.
a = No analyte response detected. Sample is rejected.
b = Outlier value per the Dixon Test, value is not included in statistical calculations.
No substantial matrix effects
A lack of substantial matrix effects is highly important for any LC-MS assay. In this validation, low and high QCs were prepared in six individual lots of human serum as well as in a lot of hyperlipemic serum and a lot of hemolyzed serum. Choice of evaluation of QCs over matrix factor was to test a more stringent approach in evaluating nSMOL as these QC lots could have differing characteristics in assay capture and trypsin digestion, as well as the typical lot to lot variation in ion suppression/enhancement caused by endogenous compounds present with chromatographic elution of the analyte.
For the matrix effects experiments, at least two-thirds of the individual lots were within 20 % of nominal concentration and the overall mean concentration was within 20 %, indicating that there were no substantial matrix effects (table 2). In addition, both hemolyzed and hyperlipemic lots were within 20 % deviation from nominal concentration (table 3). Since some individual lots were outside 20 % and since there was a bias in the hyperlipemic lot measured concentrations, some chromatographic adjustment or sample cleanup modification might be useful to improve assay performance in this area.
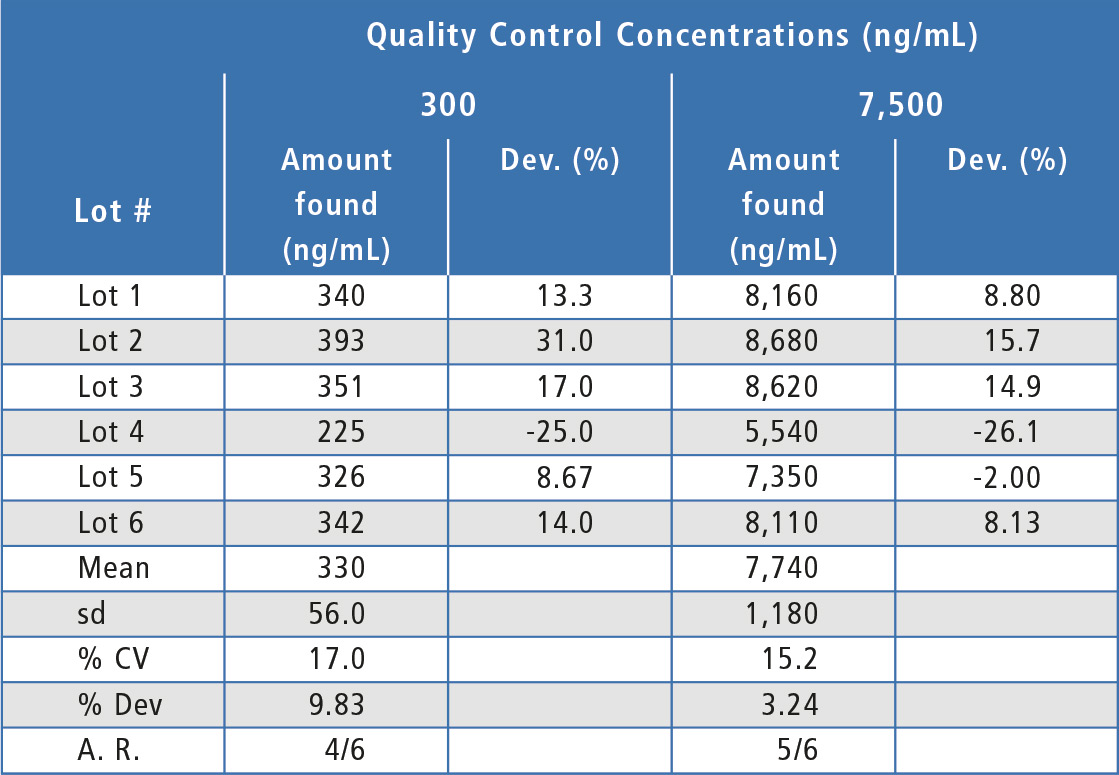 Table 2: Matrix effects for bevacizumab in individual lots of human serum. A.R. = Acceptance Ratio.
Table 2: Matrix effects for bevacizumab in individual lots of human serum. A.R. = Acceptance Ratio.
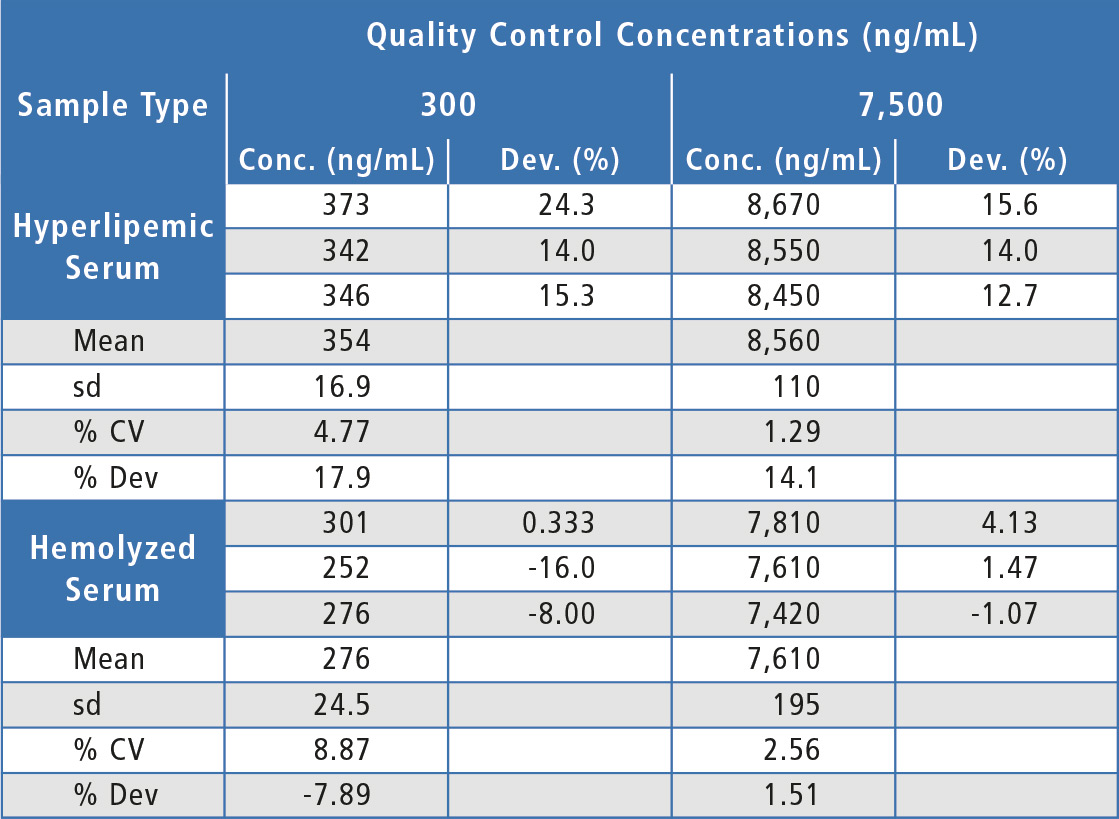 Table 3: Matrix effects for bevacizumab in hemolyzed and hyperlipemic lots of human serum
Table 3: Matrix effects for bevacizumab in hemolyzed and hyperlipemic lots of human serum
Conclusion
Overall, the nSMOL methodology resulted in acceptable assay performance in the validation of bevacizumab in human serum with minimal adjustment of standard assay conditions. As individual laboratories use nSMOL, some adjustment of assay conditions might be warranted and as with any LC-MS assay, use of a stable labeled internal standard is highly recommended.
nSMOL is a trademark of Shimadzu Corp.
Authors
Michael H. Buonarati, Ph.D., Senior Director Intertek Pharmaceutical Services
Dale Schoener, Scientific Director Intertek Pharmaceutical Services
References
[1.] Iwamoto N., Umino Y., Aoki C., Yamane N., Hamada A., Shimada T. Fully validated LC-MS bioanalysis of Bevacizumab in human plasma using nano-surface and molecular-orientation limited (nSMOL) proteolysis. Drug Metab Pharmacokinet. 31 (2016), 46-50.
[2.] Iwamoto N., Takanashi M., Umino Y., Aoki C., Hamada A., Shimada T. Application of nano-surface and molecular-orientation limited proteolysis to LC-MS bioanalysis of Cetuximab. Bioanalysis. 8 (2016), 1009-20.
[3.] Guidance for Industry: Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, May 2018.
[4.] Jenkins R., Duggan JX., Aubry AF., Zeng J., Lee JW., Cojocaru L., Dufield D., Garofolo F., Kaur S., Schultz GA., Xu K., Yang Z., Yu J., Zhang YJ., Vazvaei F. Recommendations for validation of LC-MS/MS bioanalytical methods for protein biotherapeutics. AAPS J. 17 (2015), 1-16.
More information at www.shimadzu.eu/nsmol-antibody-ba-kit