What’s in the water?
Water analysis of humic acid with fluorescence spectroscopy
Water is the basis of all life, all of nature and its habitats. When processed into food as drinking water, it’s a substance that is strictly tested. However, water also has an important task in chemistry since it is a good solvent and absorbs many substances.
The most common way of water to enter the natural cycle is as rain into the ground and then into water bodies or deeper wells. It absorbs soil components and carries them in solution or as suspended particles. There are both visible and invisible portions.
 Figure 1: Autumn view of a city park with a pond supplied with fresh water
Figure 1: Autumn view of a city park with a pond supplied with fresh water
Visible particles usually give the water an earthy color, an appearance that is normal for outdoor ponds or lakes (figure 1). However, if a slight tint in a bottle of drinking water is found, it is perceived purely subjectively as deviating from norm and experience. Drinking water is expected to be colorless and clear.
The warm summer of 2018 brought a supply of still mineral water with a slight tint (figure 2). This had to be investigated with fluorescence spectroscopy.
 Figure 2: Still mineral water with a slight coloration from a delivery in summer 2018
Figure 2: Still mineral water with a slight coloration from a delivery in summer 2018
It is known from the literature that water can contain humic acid, a natural degradation product of organic sources such as leaves and grasses. This occurs naturally with open water, and the acid serves as food for living organisms in the water. Humic acid is not a stable molecule. It oxidizes through oxygen and decomposes chemically into tryptophan and L-tyrosine. It could be deduced from this reaction that if no more humic acid is present, the necessary amount of oxygen in the water is also consumed [1], [2], [3].
In the following application, it was interesting to determine the change in humic acid content by fluorescence spectroscopy. Humic acid, tryptophan and tyrosine show a fluorescence spectrum under excitation in the ultraviolet range. Due to their chemical structure, the complex molecules have many ring systems, and individual electrons can be excited to energetically higher states. The energy absorbed in this way is released again via radiation of photons when the electron returns to its energetic ground state.
For this fluorescence analysis a rapid screening was used, ending in an EEM view (Excitation Emission Matrix). Fluorescence spectroscopy is a very sensitive and selective measurement technique. It can make traces of fluorescent-active substances visible in a mixture.
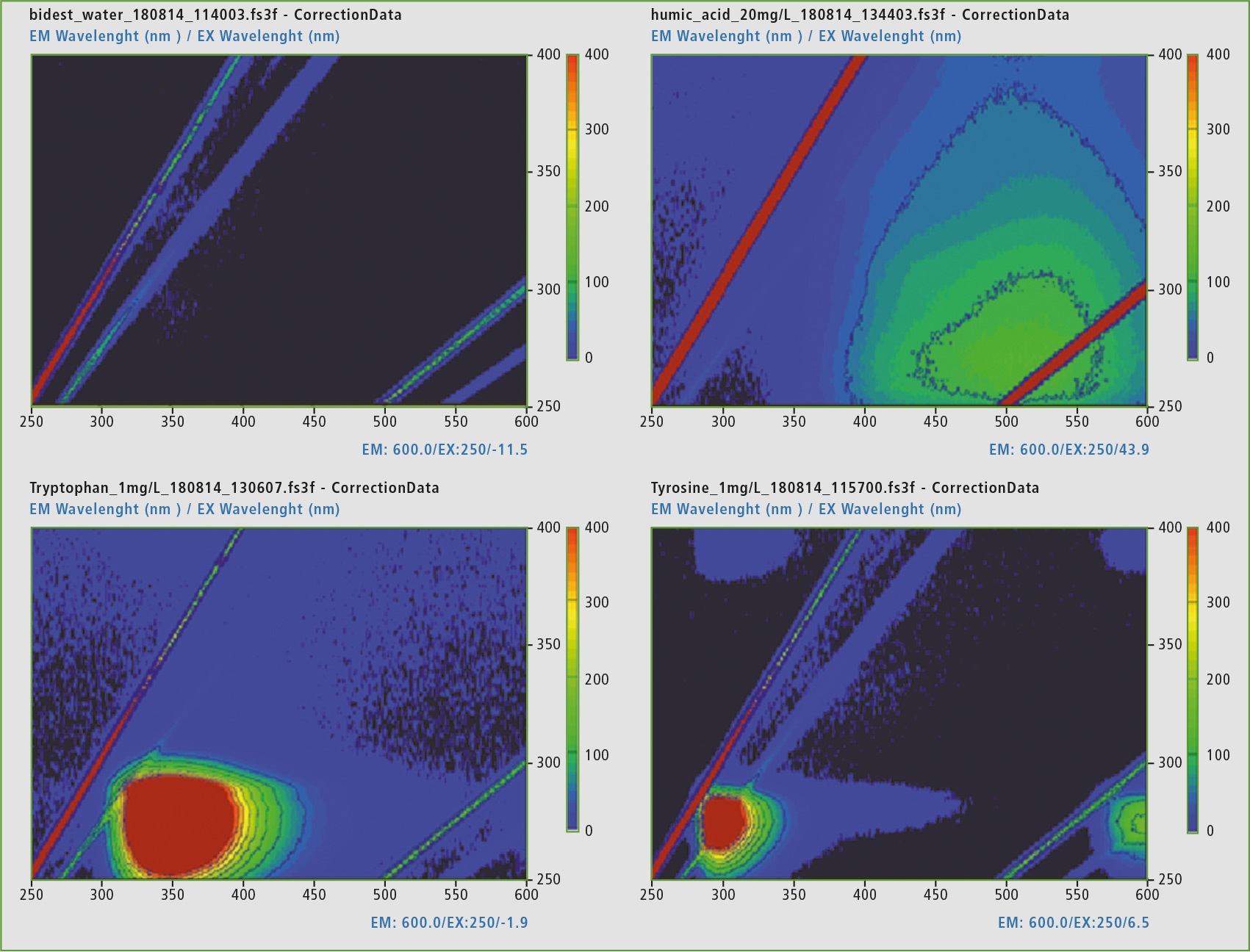 Figure 3: Representation of the EEM matrix of double distilled water (top left), humic acid (top right), tryptophan (bottom left) and L-tyrosine (bottom right), the scaling of the representation was set to 0 – 400 intensities (red corresponding to very intense, black up to no intensity).
Figure 3: Representation of the EEM matrix of double distilled water (top left), humic acid (top right), tryptophan (bottom left) and L-tyrosine (bottom right), the scaling of the representation was set to 0 – 400 intensities (red corresponding to very intense, black up to no intensity).
Samples
Various samples were collected for this study:
- A chemically pure water – double distilled,
- Well water, standing, from a garden hose,
- Well water, freshly pumped, from a garden hose,
- Pond water standing,
- Pond water with fresh water exchange, and
- Mineral water without carbon dioxide (still).
Reference materials were solutions of tyrosine (1 mg/L), tryptophan (1 mg/L) and humic acid (20 mg/L, pH 8 to 9).
All liquids used were transferred into a fluorescence cell. In the standard version, the cuvettes have four polished windows and a layer thickness of 10 x 10 mm. The quartz in this cuvette is fluorescence-free. The windows are needed because fluorescence is a scattered light which is detected at an angle of 90 degrees from the irradiation direction of the excitation source. The reference material was balanced and diluted with double distilled water. This water was also tested to exclude possible contamination (see figure 3).
Analysis of DOM in natural water
“Dissolved Organic Matter” was investigated with the Shimadzu RF-6000 fluorescence spectrophotometer. In a quick screening, EEM matrices of the samples and references were created. The EEM matrix is formed when the excitation wavelengths are increased slowly in small steps and the respective fluorescence spectrum is applied. Depending on the recording speed, it can take approx. 2 minutes (60,000 nm/s) or approx. 13 minutes (2,000 nm/s) to create the matrix.
From the reference measurements, it was possible to obtain the analytical wavelengths for the identification of the proteins involved. Distilled water shows no presence of a fluorophore, as was to be expected.
The excitation wavelength (EX) at 275 nm and the emission wavelength range (EM) at 340 – 381 nm were found for tryptophan. For L-tyrosine the EX are at 275 nm and EM at 310 – 320 nm. The humic acid has two active surfaces in the EEM at EX 300 – 370 nm and EM 400 – 500 nm, as well as EX 240 – 260 nm and an EM of 450 – 500 nm.
In the same way, the five samples from different sources were examined. All samples had lower concentrations than the reference material. It became apparent (figures 4 and 5), that the mineral water contained a low concentration of a molecule similar to humic acid. In the standing waters, the signal group of humic acid has decreased strongly. Tryptophan and L-tyrosine-similar substances predominate. Both fresh waters, on the other hand, contain mostly proteins similar to humic acid. Table 1 shows the detected intensities of all samples and references involved to make the quantitative aspect visible.
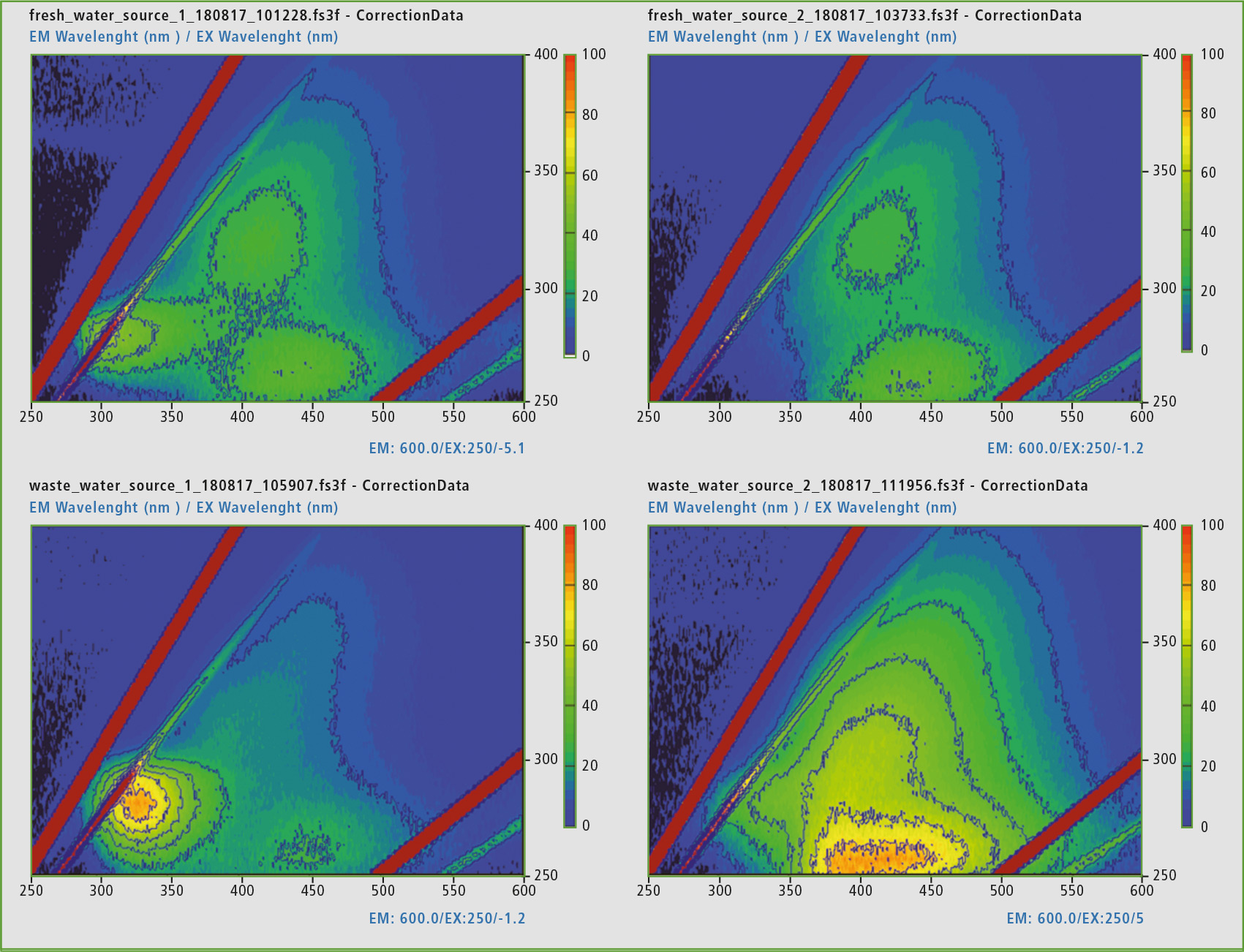 Figure 4: Representation of the EEM matrices of standing well water in a hose (top left), flowing well water from a hose (top right), standing pond water (bottom left) and flowing pond water (bottom right).
Figure 4: Representation of the EEM matrices of standing well water in a hose (top left), flowing well water from a hose (top right), standing pond water (bottom left) and flowing pond water (bottom right).
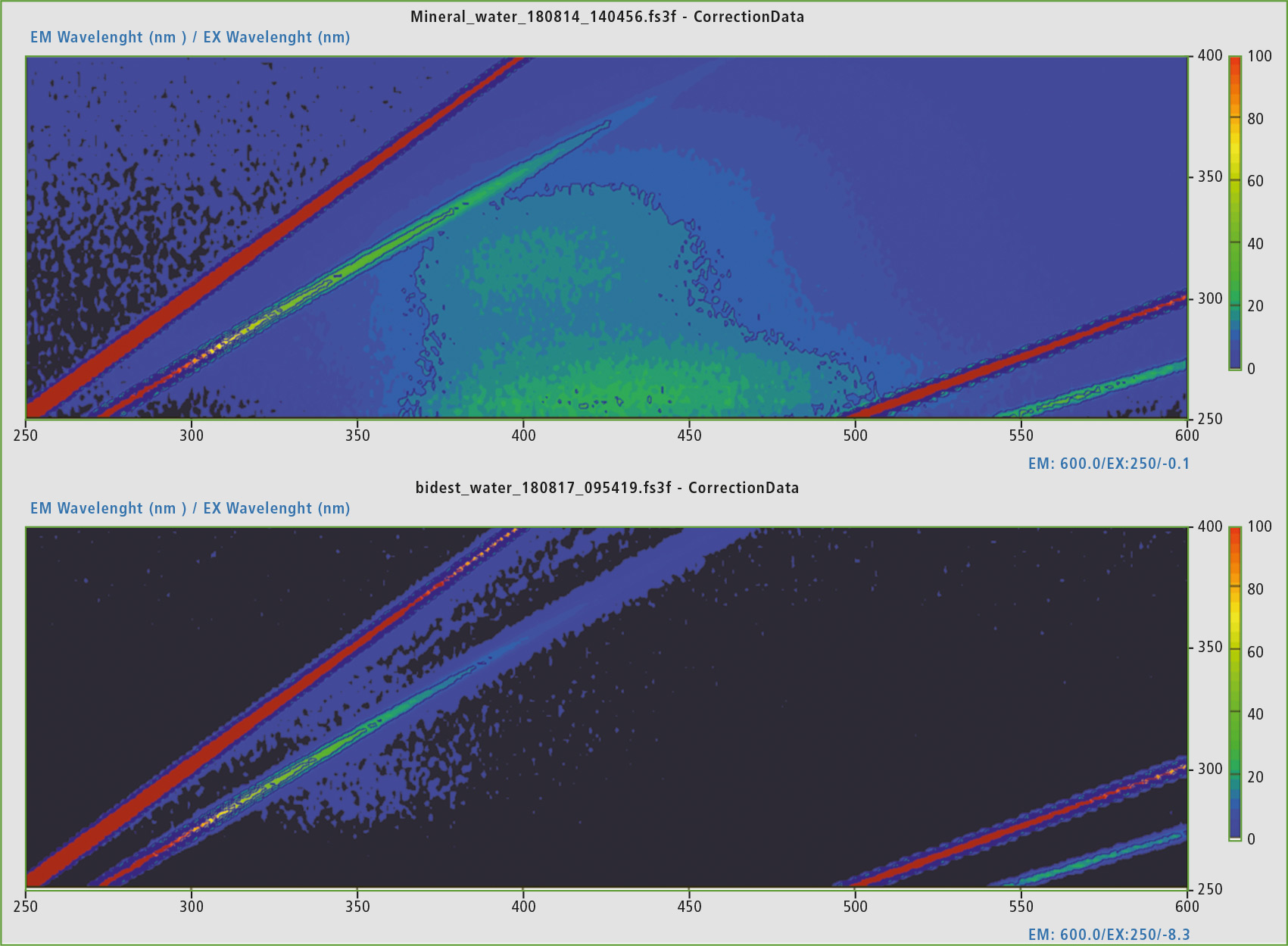 Figure 5: Comparison of the EEM matrices of still mineral water from the bottle (summer 2018) and double-distilled water from the laboratory. The mineral water exhibits fluorescence activity in the area of humic acid-like proteins.
Figure 5: Comparison of the EEM matrices of still mineral water from the bottle (summer 2018) and double-distilled water from the laboratory. The mineral water exhibits fluorescence activity in the area of humic acid-like proteins.
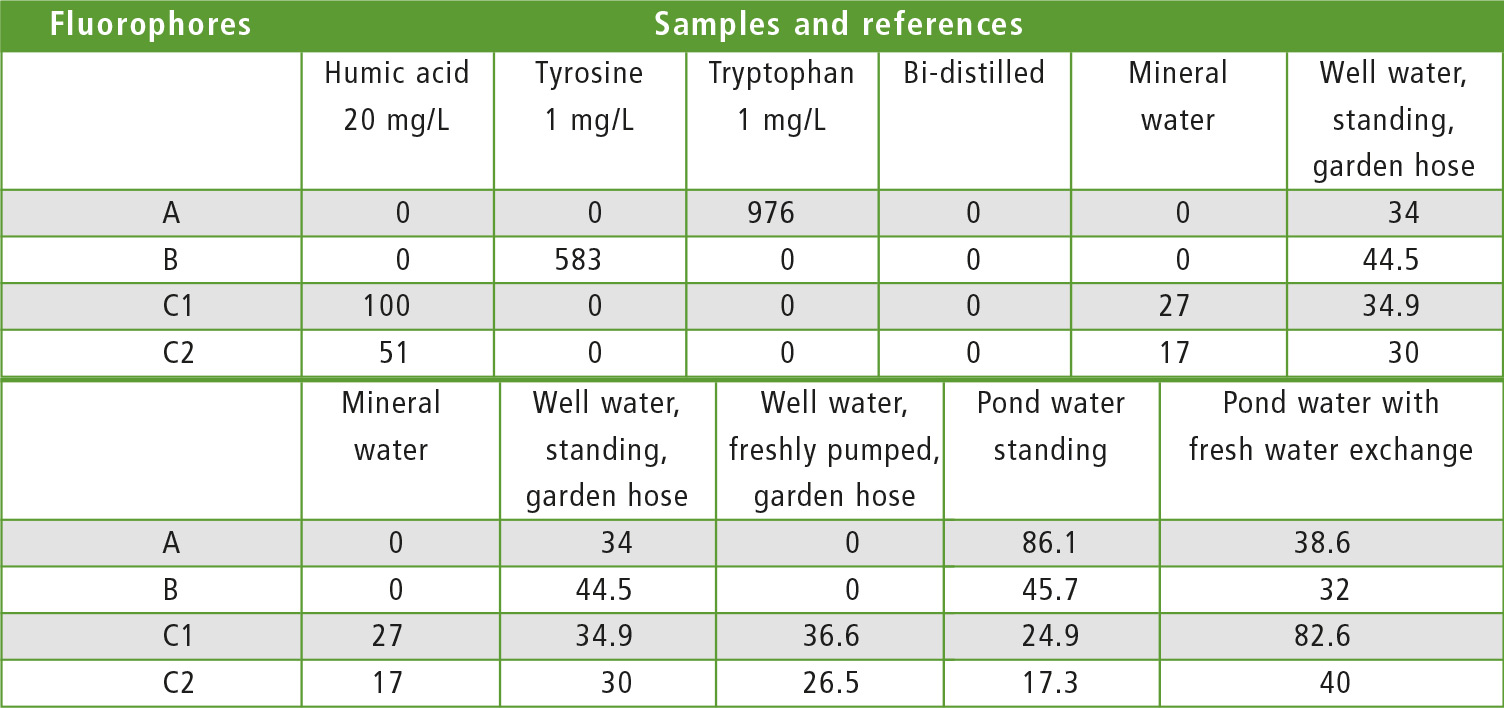 Table 1: Intensities of the fluorophores in the samples and references
Table 1: Intensities of the fluorophores in the samples and references
Conclusion
The Excitation Emission Matrix (EEM) of fluorescence spectroscopy is a very fast technique for obtaining an overview of dissolved organic components (DOM) in water. With this option, the water quality can be monitored, for example, in wastewater treatment processes and also for contamination of natural water.
Literature
[1] Hudson, N., Baker, A. and Reynolds, D. (2007). Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters – a review. River Research and Applications 23: 631-649.
[2] Yan, Y., Li, H. and Myrick, M. L. (2000). Fluorescence fingerprint of waters: excitation-emission matrix spectroscopy as a tracking tool. Applied Spectroscopy 54(10): 1539-1542.
[3] AD-0133, Shimadzu Asia Pacific, 2016
Further information on this article:
• Application: AD-0133: Excitation-Emission Matrix (EEM) Fluorescence Spectroscopy for Analysis of Dissolved Organic Matter (DOM) in Natural Water and Wastewaters
• Application: SCA_105_010: Dissolved organic matter analysis (DOM) and its appearance under different environmental conditions – fluorescence EEM matrices of different sources