An acidic challenge
Online TOC determination in concentrated hydrochloric acid

Cleaning and disinfection agents, pesticides, pharmaceuticals or plastics such as PVC – they all consist of chlorine compounds and have meanwhile become indispensible. Chlorine is one of the most important basic chemicals in the chemical industry.
Various processes for the production of chlorine, such as chlor-alkali electrolysis, have become established. The membrane electrolysis process is used about in 2/3 of commercially run plants, since the end-products chlorine, hydrogen and sodium hydroxide (NaOH) occur with almost the same purity as when using the amalgam electrolysis process – yet a clearly lower energy input is required. In addition to the membrane process, hydrochloric acid electrolysis according to the ODC (Oxygen Depolarized Cathode) process has become established.
Hydrochloric acid is generated as a by-product in some processes and is, therefore, present in high amounts. The membranes used are sensitive to certain contaminations in the hydrochloric acid, such as organic compounds. This is why it is important to determine if hydrochloric acid contains organic contaminations prior to its use. Quality assurance also plays an increasingly important role in the sale of hydrochloric acid.
TOC – the sum parameter
The TOC (Total Organic Carbon) sum parameter is a measure of contamination by organic compounds. It provides an indication of how much of the carbon present in the sample originates from organic compounds.
Specialized TOC analyzers are used for this purpose. The sample is acidified in the analyzer in order to destroy inorganic carbon compounds present, such as carbonates and hydrogen carbonates. The resulting carbon dioxide is subsequently removed using a sparging gas.
An aliquot of the pretreated sample is injected onto a heated catalyst (680 °C). The organic compounds are converted to CO2 and detected via an NDIR detector.
In addition to conventional laboratory analysis, TOC determination can be simply and securely implemented online. Such process analyzers operate autonomously in close proximity to the sample stream to be analyzed. Depending on the parameter setting, they take a sample automatically every few minutes, prepare and analyze it and send the results to a control station or issue a warning when a limit value is exceeded.
 Figure 1: Concentrated hydrochloric acid is diluted 1:3
Figure 1: Concentrated hydrochloric acid is diluted 1:3
TOC in concentrated hydrochloric acid
These types of process analyzers are used routinely in many application areas, such as wastewater monitoring. Online TOC monitoring of concentrated hydrochloric acid, however, remains a major challenge.
As the extremely acidic sample does not contain any hydrogen carbonates, the sample preparation step (acidification and sparging) can be omitted. Reliable TOC determination in concentrated hydrochloric acid is, nevertheless, anything but easy. In close cooperation with the engineering department of a Shimadzu costumer, a project was realized determining TOC in hydrochloric acid.
Simultaneous TN determination
A major advantage of catalytic combustion oxidation for the determination of TOC is the possibility to simultaneously detect the amount of nitrogen compounds. During combustion, the nitrogen compounds are converted to nitrogen monoxide (NO). A chemoluminescence detector (CLM) is connected in series following the CO2 selective NDIR detector. The incoming NO is oxidized to nitrogen dioxide (NO2) using ozone, and the photons emitted during this reaction are detected by the CLM detector. This combination of detectors enables simultaneous detection of TOC and nitrogen compounds.
 Figure 2: TOC-4200 in a protective cabinet
Figure 2: TOC-4200 in a protective cabinet
Protection and safety
To protect the analyzer from aggressive hydrochloric acid fumes, various gas washers are needed. Shimadzu’s TOC-4200 is equipped with several such gas washers that chemically bind the aggressive chlorine gas.
Also needing to be considered in this analysis, is that hydrochloric acid is a gas being released from a concentrated solution. The concentrated hydrochloric acid must therefore be diluted prior to reaching the analyzer. This requires a dilution apparatus located outside of the analyzer (figure 1), which dilutes concentrated hydrochloric acid 1:3 with ultrapure water.
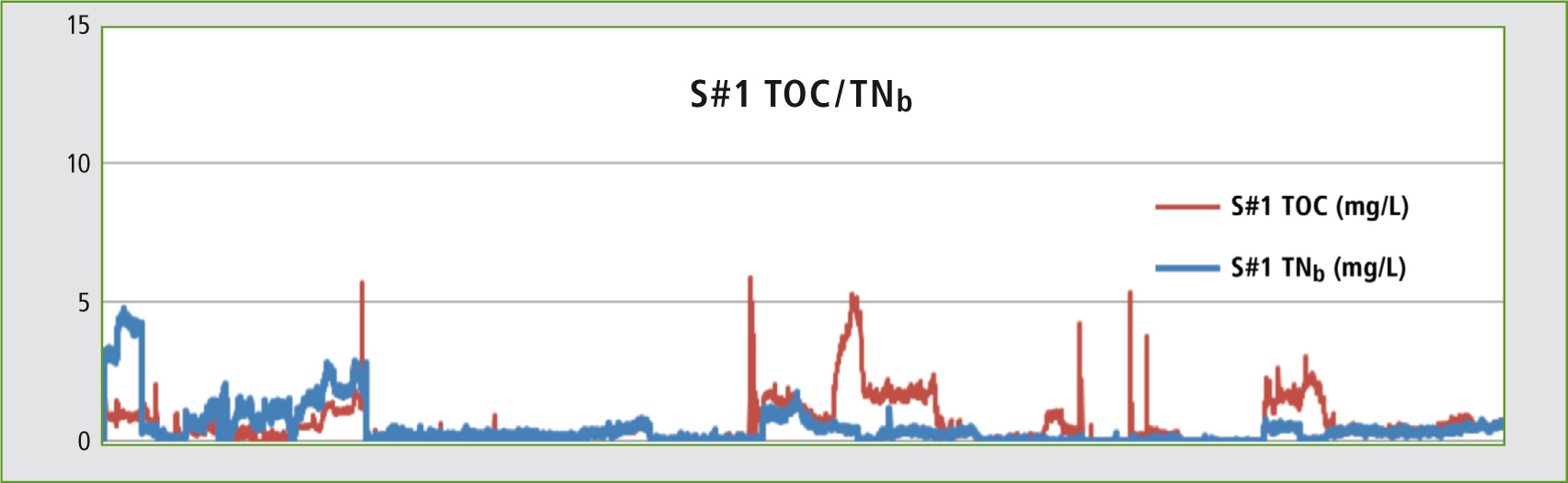 Figure 3: Trend graph: TOC and TNb -results measured over a period of four months
Figure 3: Trend graph: TOC and TNb -results measured over a period of four months
Another safety issue is occupational safety. Employees should not come into contact with aggressive vapors. This is why these types of analyzers must be surrounded by a protective cabinet equipped with hydrochloric acid sensors. An alarm signal is issued when HCl (hydrogen chloride) gas is detected. This protects employees as well as materials.
Taking these issues into account, a reliable system for the determination of organic load in hydrochloric acid is designed. And what has long been a routine laboratory application can now also be analyzed online.
In a test phase, concentrated hydrochloric acid was monitored over a period of four months. The TOC as well as the TNb was calibrated over a range of 1-10 mg/L. The TOC-4200 features an automatic dilution function enabling creation of a multi-point calibration from a standard solution. As the acids need no further acidification, sample preparation (acidification and sparging) is not necessary. The injection volume is 50 µL.