New HPLC-FAAS hyphenated technique for determination of aluminium and aluminium fluoride complexes

Dr. Marcin Frankowski, Department of Water and Soil Analysis, Faculty of Chemistry, Adam Mickiewicz University
Aluminium as an element commonly found in the earth’s crust (8 % by weight) and characterized by strong amphoteric nature [1], can create numerous complex species [2]. The form in which aluminium occurs in the environment affects its mobility, bioavailability and toxic influence on living organisms and vegetation. Toxicity of aluminium is mainly connected with the occurrence of a free Al3+ ion, hydroxy forms (including Al(OH)2+, Al(OH)+2) and inorganic form of complexes. Among inorganic forms, aluminium fluoride complexes dominate [e.g. 3,4]. They are characterized by high values of stability constant (AlF2+, Log K = 12,600; AlF2+, Log K = 7,000).
The forms of aluminium fluoride complexes (AlF2+, AlF2+, AlF30, AlF4–, AlF52-, AlF63-) and their occurrence depend on pH and ligand concentration in solution [e.g. 5]. It should be emphasized that determination of only the total concentration of aluminium does not provide full data concerning the processes the element undergoes in the natural environment and consequently, does not provide information on migration, actual toxicity, bioavailability and cumulation in particular components of the environment.
One of the best known and most commonly applied procedures of speciation analysis is the Driscoll’s method, which enables isolation of a fraction of labile monomeric inorganic aluminium containing an Al3+ ion and bonds with inorganic fluoride and sulphate ligands [e.g. 6]. However, this method does not enable direct determination of particular speciation forms of aluminium, including fluoride complexes and Al3+, Al(OH)2+, Al(OH)+2) species.
HPLC method for aluminium fluoride analysis
The use of liquid chromatography provides many possibilities to separate particular forms of aluminium, both cation and anion. Bertsh and Anderson [7] were among the first researchers who suggested the separation of aluminium fluoride complexes applying ion chromatography. The AlF2+ and Al3+ forms were separated. The method was not used in environmental samples determination. Also Willet [8] did not obtain a good separation of AlF2+ and AlF+2 forms, despite the quick separation. Motellier and Pitsch [9] did not achieve proper resolution for the first two peaks AlF2+ and AlF+2. So far, research on speciation of AlFx complexes using ion chromatography has been based on isocratic elution.
Bormann and Seubert [10] first used UV spectrophotometry and atomic spectrometry ICP-AES for Al-citrate-oxalate complexes. The signals obtained were assigned to the following forms: AlF+2, AlF2+ and Al3+. To date, the combination of HPLC with ICP (mass spectrometry and atomic emission spectrometry (ICP-MS, ICP-AES) in online as well as offline systems has been used. The researchers have also applied atomic absorption spectrometry with electrothermal atomization in the offline system.
The first online systems based on HPLC and FAAS detection were developed by Ziola-Frankowska et al. [11] and Frankowski et al. [12]. These analytical systems encountered some problems with quantitative analysis. Signals from the detector were collected in three replicates for 30 sec. (90 sec. software limited), and absorbance values were then counted manually every 0.5 sec. and saved as a txt file, which was exported to another software for calculation of peak area. However, this method is time-consuming and requires additional tools.
New HPLC-FAAS system
Requirements of high sensitivity and the most universal analytical system for soil and water analysis are known to be very high. A new system has to be capable of separating and determining inorganic as well as organic compounds. On the other hand, there is a demand for the most automated system possible, utilizing different analytical techniques in one run.
Determination of ion complexes in water, use of a chromatographic technique to separate and on-line atomic absorption technique for detection of concentrations of complexes seem to be the most promising solution. A possible additional advantage of this solution is an increase in analytical throughput.
The experimental system consisted of Shimadzu’s LC-10 liquid chromatograph and the new AA-7000 flame atomic spectrometer. The Shimadzu CBM-20A communication module and LabSolution software were the key point in interfacing these two systems. The electronic interfacing necessary was configured in SEG Shimadzu, Duisburg, Germany. The chromatographic separation procedure along with the optimization of the complete HPLC-FAAS system have already been described [11, 12] and are also presented in table 1. The HPLC-FAAS system is presented in figure 1.
 Figure 1: The HPLC-FAAS analytical system used in the laboratory of Department of Water and Soil Analysis, Faculty of Chemistry, Adam Mickiewicz University, Poznan, Poland
Figure 1: The HPLC-FAAS analytical system used in the laboratory of Department of Water and Soil Analysis, Faculty of Chemistry, Adam Mickiewicz University, Poznan, Poland
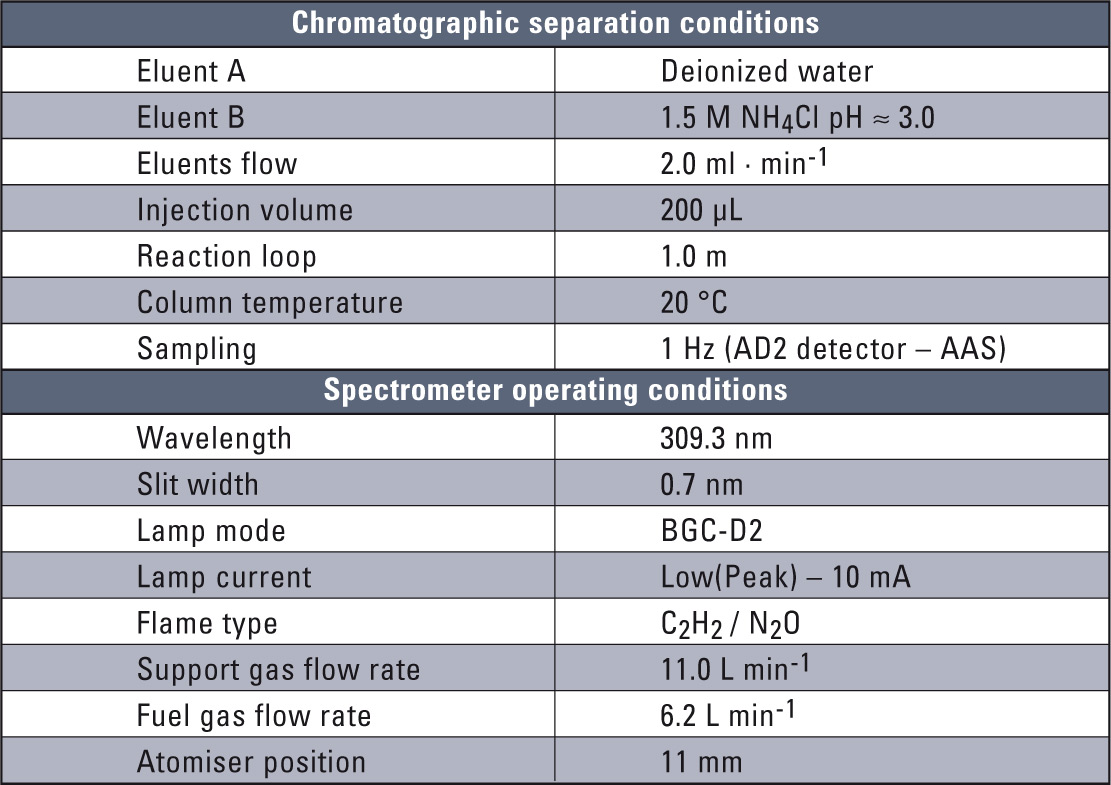 Table 1: Basic chromatographic and spectroscopic conditions
Table 1: Basic chromatographic and spectroscopic conditions
Analytical results
The single analysis by means of the solution presented takes four minutes and does not require post-column treatment. The column effluent was connected directly to the AA spectrometer nebulizer using a capillary tube.
Linearity of the tested system is presented in figure 2. The coefficients obtained were; r2 = 0.996 and r = 0.998 (Figure 3).
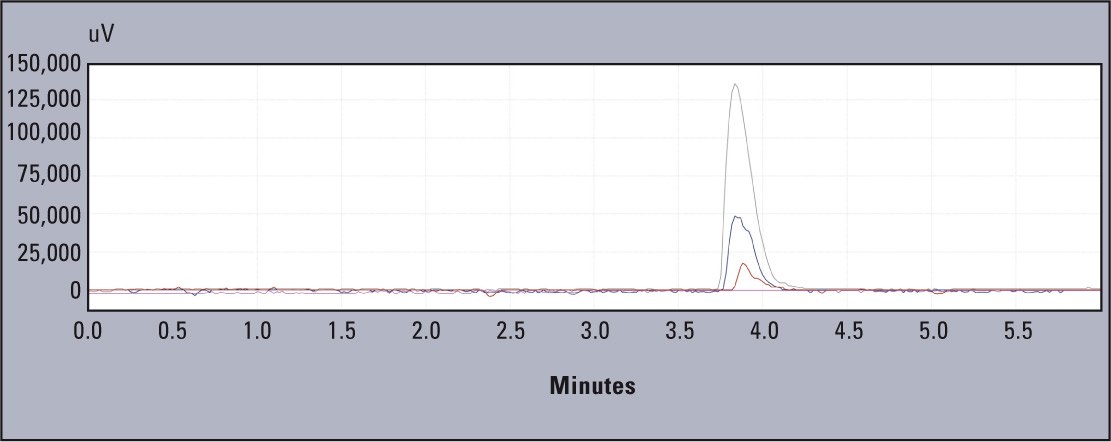 Figure 2: Overlapping chromatograms for standards 10, 50 and 100 mg L-1 of aluminium standard solution (Al in HNO3, Merck)
Figure 2: Overlapping chromatograms for standards 10, 50 and 100 mg L-1 of aluminium standard solution (Al in HNO3, Merck)
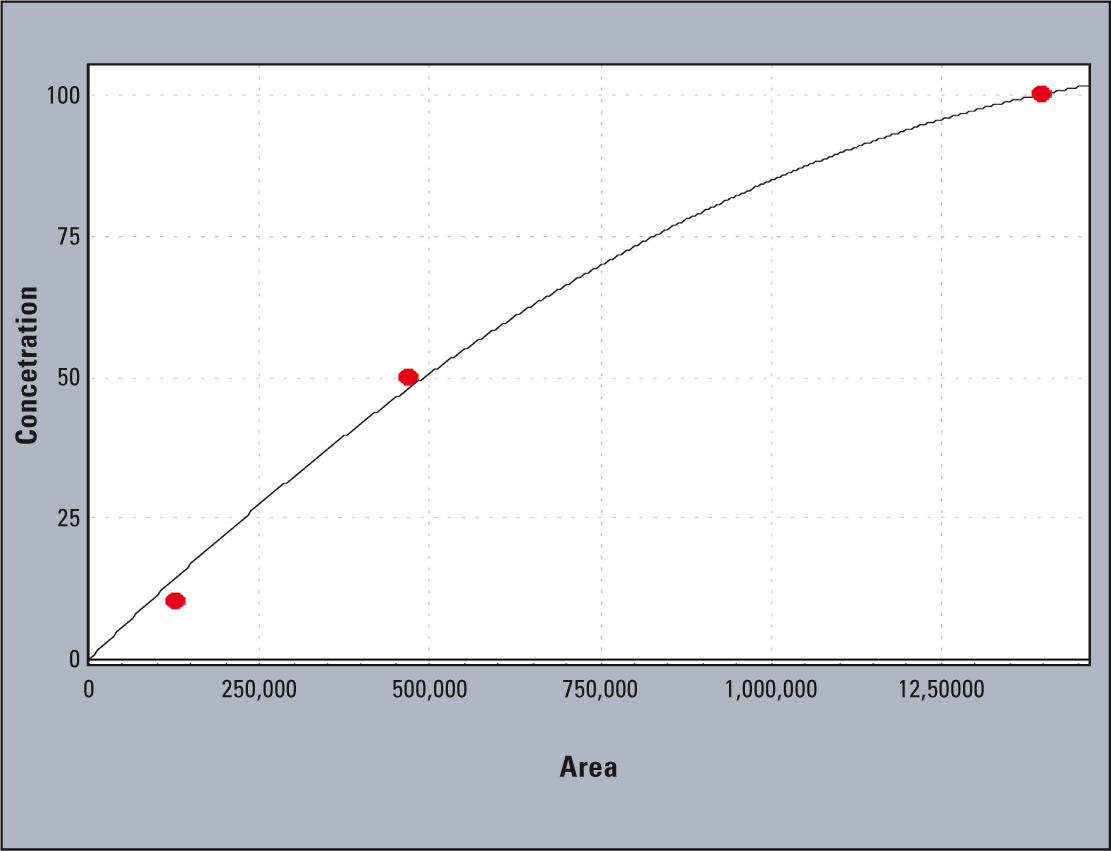 Figure 3: Calibration curve for the sample standard solutions analyzed: 10, 50, 100 mg L-1 of aluminum standard solution (Al in HNO3, Merck)
Figure 3: Calibration curve for the sample standard solutions analyzed: 10, 50, 100 mg L-1 of aluminum standard solution (Al in HNO3, Merck)
Chromatograms of three different real samples are presented in table 2. The analysis was performed for characteristic solutions with variable concentration of aluminum ion in relation to ion fluorides, so the system enabled observation of the influence of the particular forms of aluminium.
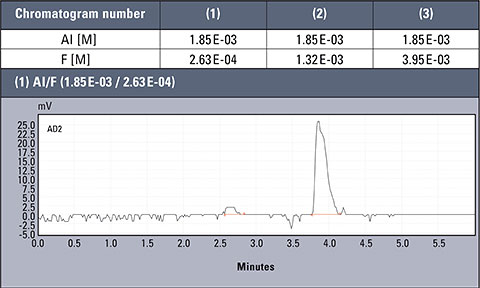
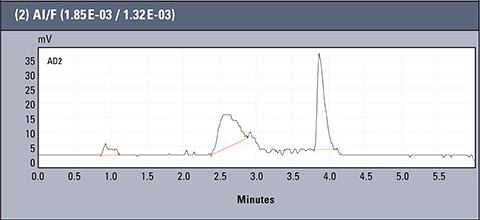
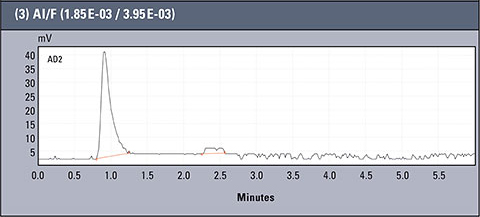 Table 2: Concentration values of Al and F- for AlFx(x-3)
Table 2: Concentration values of Al and F- for AlFx(x-3)
The quantitative results obtained comply with the reference results acquired during analysis of the large number of samples of standard model solutions [11, 12]. The proposed elution sequence is presented in table 3.
 Table 3: Proposed elution sequence of ionic forms: AlF2+, AlF2+, AlF30, AlF4–, Al3+ *AlF30 – possible elution with +1 and -1 AlF Typen (forms (depending on the ratio Al:F)
Table 3: Proposed elution sequence of ionic forms: AlF2+, AlF2+, AlF30, AlF4–, Al3+ *AlF30 – possible elution with +1 and -1 AlF Typen (forms (depending on the ratio Al:F)
A quantitative analysis carried out using FAAS technique was straightforward with application of the LabSolution chromatographic software for atomic absorption signals. This solution reduced both time of data managing and analysis while increasing accuracy and precision of the determinations. Table 4 shows results of sample signals obtained for the samples listed in table 2.
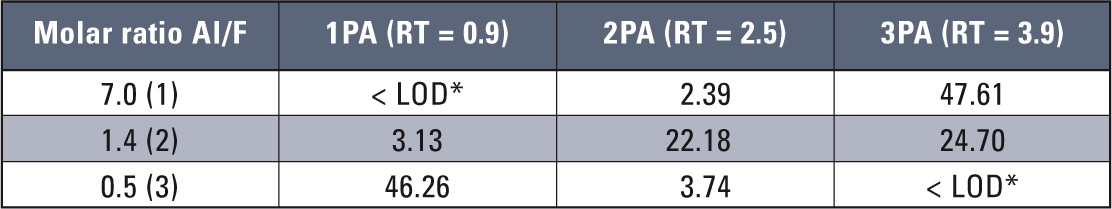 Table 4: Determined concentrations of aluminium and aluminium fluoride complexes *for loop 200 µL
Table 4: Determined concentrations of aluminium and aluminium fluoride complexes *for loop 200 µL
Conclusions
The application of HPLC-AAS system allows a fast and accurate speciation analysis of water impurities. The system allows performing of quantitative and qualitative analysis of different aluminium forms. Use of the AA-7000 with LabSolution software enabled overcoming of the usual difficulties encountered due to integration of the atomic absorption signals. The method presented can be the basis for analysis of the speciation arrangement of other elements which may be found in the natural environment.
Instrument configuration
- AA-7000 Shimadzu spectrometer with acetylene-N2O burner
- CBM-20A communication module
- PC 55L electronic interface
- LabSolution software
- Solvent delivery module LC-10 ADVP
- Flow control valve FCV-10 ALVP
- Degasser unit DGU-20A5
- Column oven CTO-10ASVPP with Rheodyne 7725i Injection Valve
- Ion-exchange column – Dionex IonPac CS5A (Analytical column, 250 mm, 4.0 mm i.d., particle size 9.0 µm)
- IonPac CG5A (guard column, 50 mm, 4 mm i.d., particle size 9.0 µm)
- Gradient at 2 ml min-1 with an injection volume of 200 µL
- Mobile phases: de-ionized water and 1.5 M NH4Cl solution (both acidified to pH≈3).
References
- A. Kabata-Pendias, H. Pendias, PWN Warsaw, 1999, pp. 192 (in Polish)
- M. Busch, A. Seubert, Anal. Chim. Acta, 1999, 399, 223
- S. Bi, X. Yang, F. Zhang, X. Wang, G. Zou, Fresenius J. Anal. Chem., 2001, 370, 984
- C. T. Driscoll, K. M. Postek, The Environmental Chemistry of Aluminium, Chapter 9, 1996, pp. 364
- A. Strunecká, O. Strunecký, J. Potoćka, Physiol. Res., 2002, 51, 557
- C. T. Driscoll, Intern. J. Environ. Anal. Chem., 1984, 16, 267
- P. M. Bertsch, M. A. Anderson, Anal. Chem., 1989, 61 (6), 535
- I. R. Willet, Soil Sci. Am. J., 1989, 53, 1385
- S. Motellier, H. Pitsch, J. Chromatogr. A., 1994, 660, 21
- G. Borrmann, A. Seubert, Anal. Chim. Acta, 1996, 332, 233
- A. Ziola-Frankowska, M. Frankowski, J. Siepak, Talanta, 2009, 78, 623.
- M. Frankowski, A. Ziola-Frankowska, J. Siepak, Talanta, 2010, 80, 2120.