Acid Test
TOC determination in concentrated hydrochloric acid
 Figure 1: Shimadzu’s TOC-L for the determination of hydrochloric acid and OCT-L autosampler for automation of the analysis.
Figure 1: Shimadzu’s TOC-L for the determination of hydrochloric acid and OCT-L autosampler for automation of the analysis.
Incoming goods control is essential in the chemical industry. The impurities present in reagents often cause impurities in products. In addition to the targeted analysis of known compounds, sum parameters can help to assess the raw chemicals with respect to their impurities. TOC (Total Organic Carbon) plays an important role here: this parameter describes the contamination by organic compounds and specifies the total amount of organic carbon. TOC can therefore be used only for the assessment of inorganic chemicals.
Acids, particularly hydrochloric acids, belong to one of the large groups of inorganic chemicals frequently used in the chemical industry. Determination of the total carbon content in concentrated hydrochloric acid presents an enormous challenge to the analyzers used. For its new TOC-L series, Shimadzu has developed an application enabling the analysis of low TOC concentrations in concentrated hydrochloric acid.
Acids – a major challenge to materials and methods
The great challenge for TOC measurements in concentrated hydrochloric acid is the development of mechanisms to protect instruments and their components, as well as to prevent damage by acid fumes. For this purpose, the TOC-L series offers several gas washers that bind and eliminate the chlorine gas formed in the flow line of the system in various ways. An additional challenge in this application is to attain a stable and reproducible oxidation so that no fluctuating or tailing peaks are being recorded. Furthermore, the measuring values should remain stable over a larger measurement period.
Low limits of detection are possible
It is usually possible to greatly dilute the substance to be analyzed, in order to eliminate matrix interferences. Sometimes however, it is necessary to attain very low limits of detection (with reference to 37 % hydrochloric acid) of 1 mg/L.
Shimadzu’s TOC-LCPH operates with catalytic oxidation combustion at 680 °C. The 37 % hydrochloric acid solution was manually diluted to 1:2 with water, in order to obtain an 18.5 % hydrochloric acid solution. Calibration (Figure 2) was carried out in the range of 0.5 to 10 mg/L. Using the automatic dilution function of the analyzer, this calibration was automatically carried out from a single stock solution. Injection volume in this case was 150 µL.
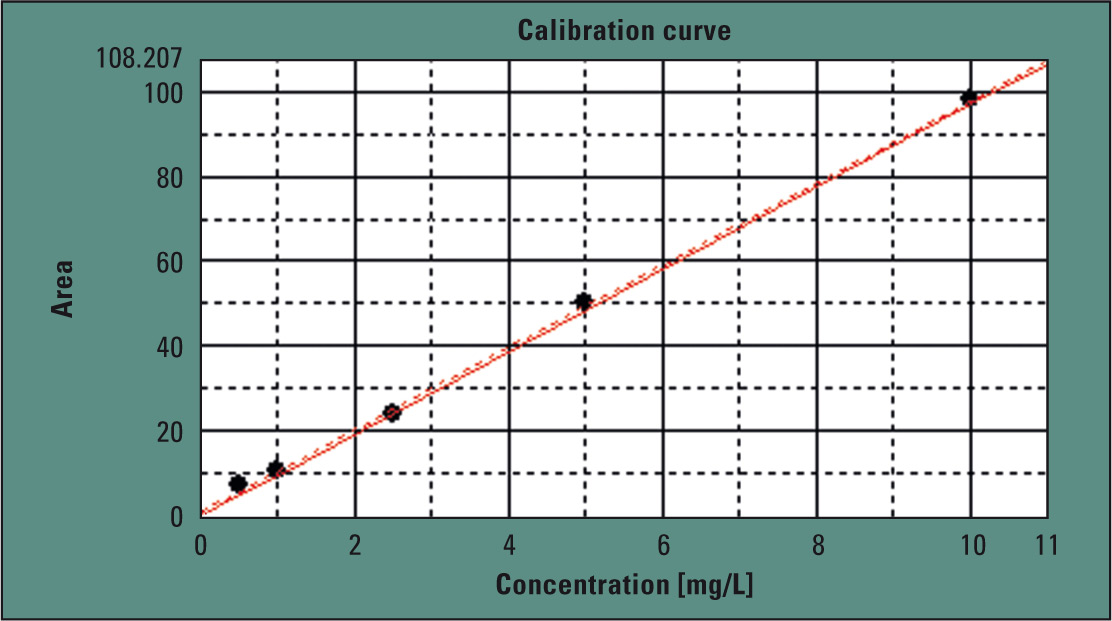 Figure 2: Calibration of the method in the range of 0.5 to 10 ppm
Figure 2: Calibration of the method in the range of 0.5 to 10 ppm
If TOC contamination of the hydrochloric acid exceeds the measuring range of the calibration, the automatic dilution function of the analyzer readjusts the hydrochloric acid solution to fit the measuring range. After calibration, the TOC content of the concentrated hydrochloric acid was determined. To investigate matrix influences, TOC content of the 18.5 % hydrochloric acid solution was subsequently doped by 5 mg/L using a potassium hydrogen phthalate solution (measuring results in Table 1).
To investigate the long-term stability of the method, the 37 % hydrochloric acid solution was again diluted by 1:2 with water and injected 76 times (150 µL). The relative standard deviation over all measurements was 3.4 %.
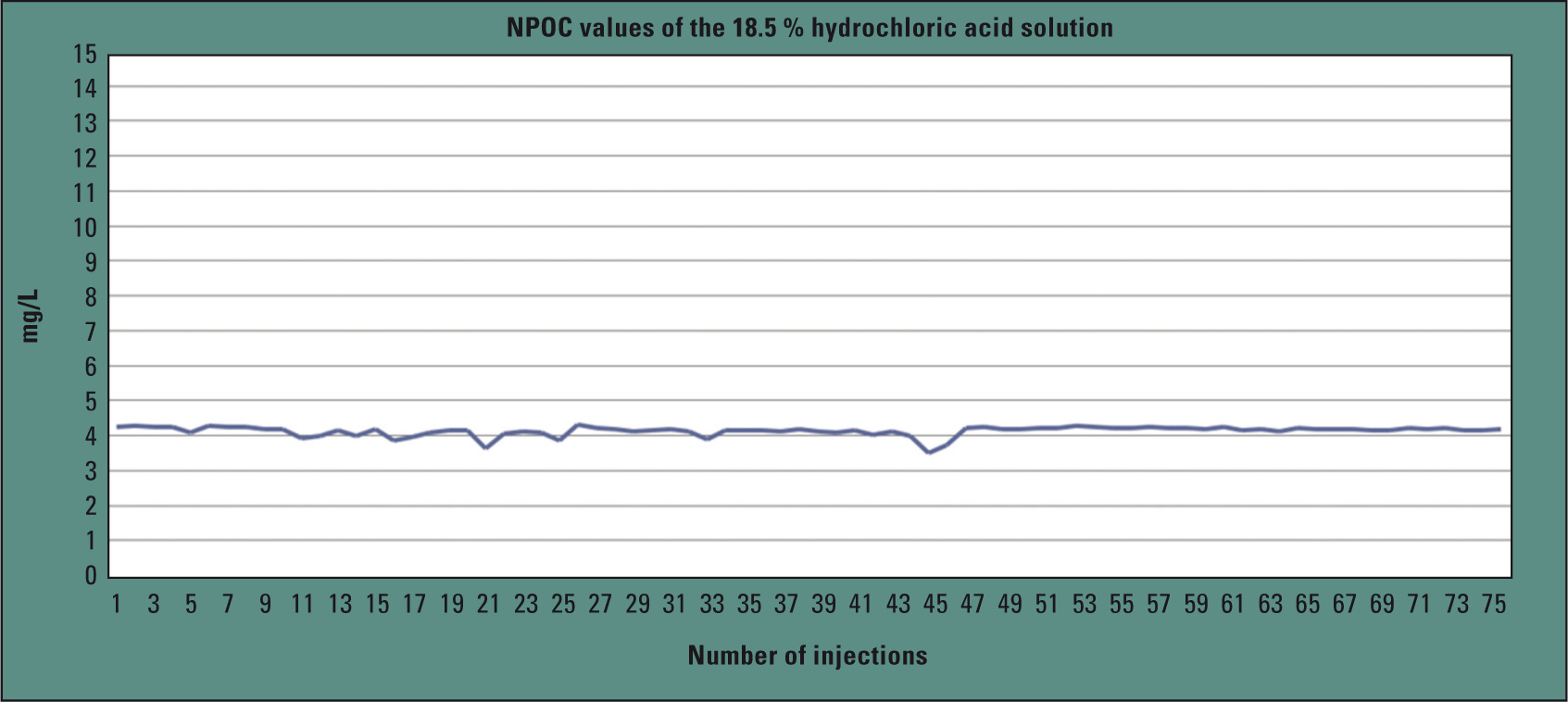 Figure 3: TOC values for 76 hydrochloric acid injections
Figure 3: TOC values for 76 hydrochloric acid injections
Figure 3 shows the progression of the TOC values for the hydrochloric acid injections. Blank values and standards (10 mg/L) were alternately measured between the individual measurements. To automate this hydrochloric acid analysis as much as possible, the TOC-L series offers an autosampler consisting entirely of inert materials, enabling the analysis of up to 16 individual sample vials.
TOC analysis of concentrated nitric and sulfuric acid
Further experiments show that the experimental setup was suitable not only for the analysis of hydrochloric acid but also for other high purity chemicals, such as concentrated nitric acid and various salt solutions. A further modification of the system also enables TOC analysis of concentrated sulfuric acid or high-percentage brines. For this purpose, an additional scrubber and a special combustion tube with a special catalyst mixture was used.
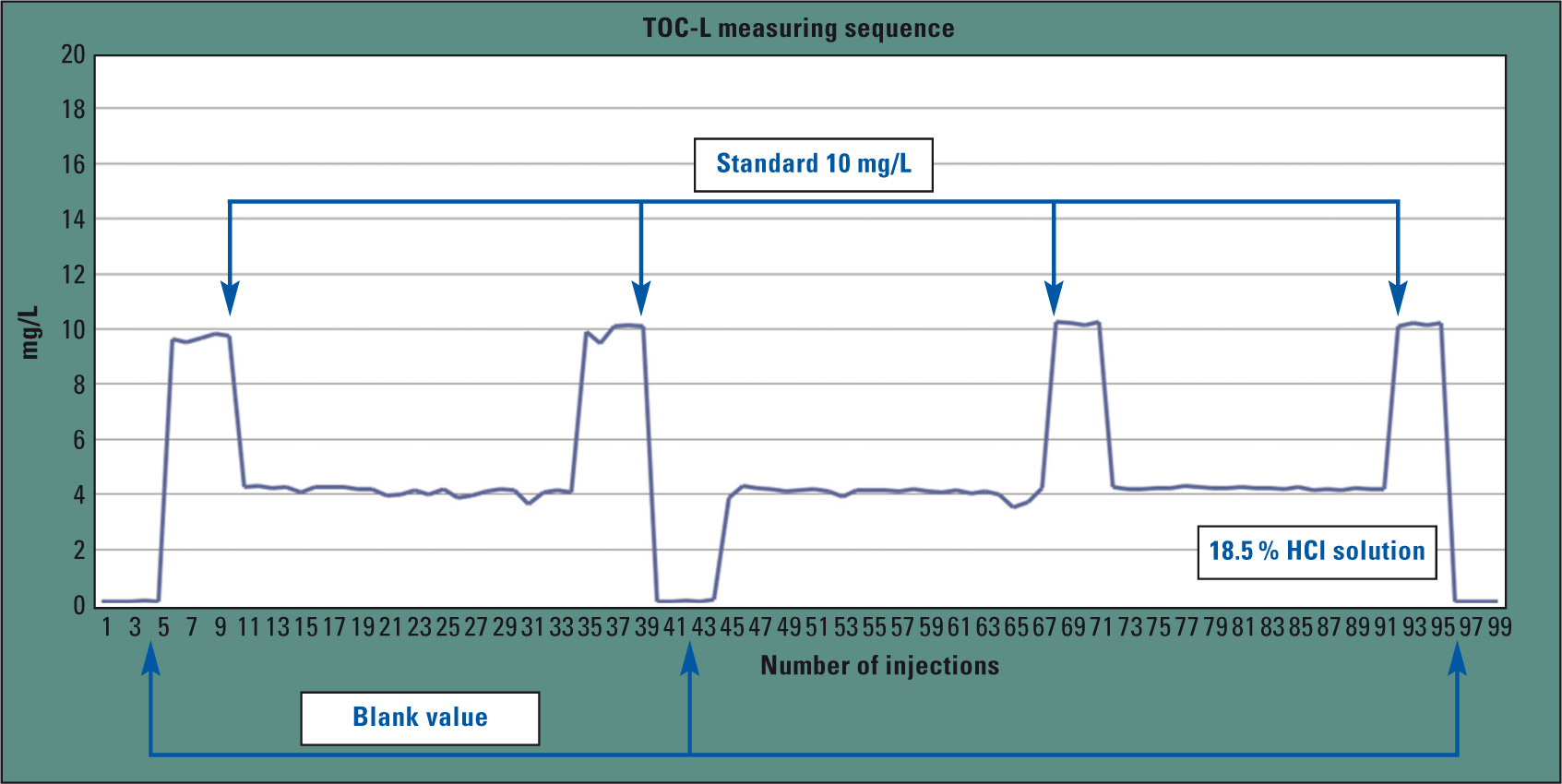 Figure 4: Sequence of a hydrochloric acid measurement. Hydrochloric acid, blank values and standards (10 mg/L) were measured alternately.
Figure 4: Sequence of a hydrochloric acid measurement. Hydrochloric acid, blank values and standards (10 mg/L) were measured alternately.
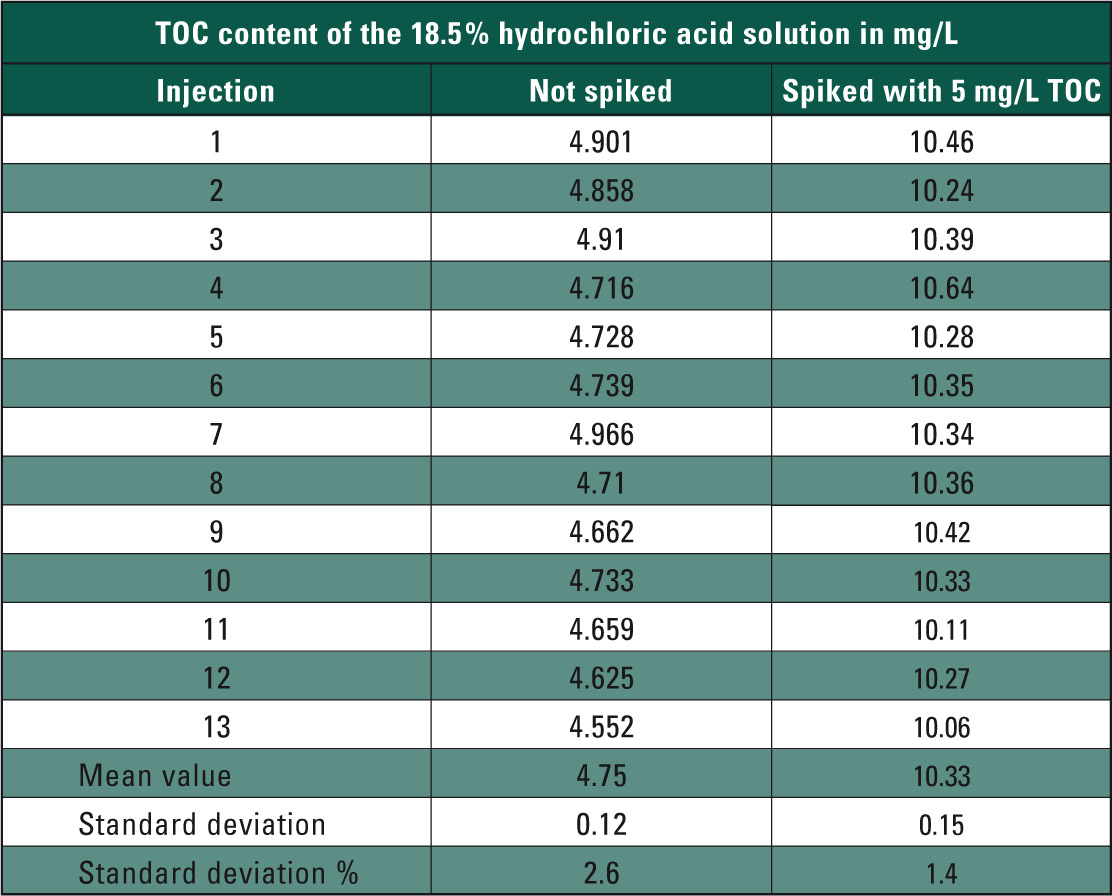 Table 1: TOC measuring values of the 18.5 % hydrochloric acid solution in mg/L
Table 1: TOC measuring values of the 18.5 % hydrochloric acid solution in mg/L