A contribution to climate protection
Analytical process control in bioethanol production
Martin Meyer, Shimadzu Europa

In light of the fight against climate change, bioethanol as a fuel has become increasingly important in recent years. However, since the raw material could also be used as animal feed or a foodstuff in most cases, bioethanol producers have a great responsibility to utilize the raw materials as efficiently as possible. Accurate control of the predominantly biological process is crucial for this. This article provides an insight into the production process from the raw material to the finished ethanol. Can technology from Shimadzu help optimize the process?
Half past eight in the morning – a busy time at gas stations. When drivers stand in front of the pump, they have a choice: E5 or E10? What many don’t know is that the E stands for “ethanol” (or simply “alcohol”), and the number indicates the amount of ethanol in that product –5 or 10 %. The EU is in the continuous process of increasing the amount of biofuels used (such as bioethanol), as the higher admixture of products obtained from renewable sources is intended to improve the CO2 balance of road traffic.
Although fuels from renewable sources also release CO2 into the environment, the plants from which they are obtained have previously absorbed a significant amount from the environment. As a result, they contribute less to CO2 pollution than fossil fuels overall. However, the production of bioethanol is not straightforward. Bioethanol is produced from plants with a high starch or sugar content.[1] This varies greatly depending on the climate and region. In European countries such as Germany and France, for example, wheat and sugar beet are used, in the USA corn (maize) and in South American countries sugar cane is the preferred option.
When it comes to producing ethanol, it is important to distinguish between raw materials that contain sugar and those that contain starch. Although starch also consists only of linked glucose molecules, additional steps are required to break down the starch and convert it into smaller sugar molecules. The enzyme alpha-amylase breaks down the starch into smaller chain pieces (polysaccharides), and the enzyme glucoamylase releases the glucose from these polysaccharides. Raw materials containing sugar, such as sugar cane and sugar beet, can be metabolized directly into ethanol using yeast fungi.
Monitoring the fermentation process
The biological process in which yeast fungi convert sugar to ethanol is known as fermentation. During this process, one molecule of glucose is chemically converted into two molecules of ethanol and two molecules of CO2.
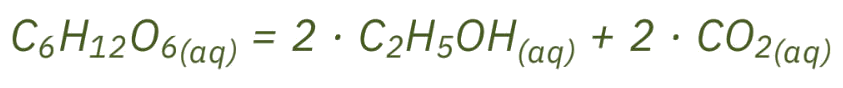
Despite the apparent simplicity of the formula, the success of the process depends on a number of critical factors, such as the composition of the raw material, temperature, time as well as the pH value. That’s why it is important to monitor the process regularly in order to make the conversion to ethanol as efficient as possible. The ability to identify the different types of sugar, determine their concentrations and control the ethanol content plays a decisive role in this regard. During the process, various organic acids can also form which, although present in small quantities, have a huge impact on the pH value. An increased content of organic acids and the resulting low pH value can stop the fermentation process altogether. It is important to keep an eye on all these factors in order to recognize potential problems at an early stage and ensure that the fermentation process runs smoothly. This is the only way to obtain the largest possible amount of ethanol from the raw material.
Determining the types of sugar poses a particular challenge, as many of these substances are very similar in terms of their chemical composition. Fructose and glucose, for example, have the same chemical formula but differ in their structures.
The analysis is further complicated by the fact that most sugars cannot be differentiated using a UV-Vis detector. Furthermore, organic acids are usually determined applying different chromatographic methods than those for sugars. Therefore, it is extremely difficult to combine and analyze all components with one single method.
With the Shim-pack SCR-102H column, Shimadzu offers a solution that enables both ion exclusion and size exclusion chromatography. The column can separate sugars and organic acids at the same time so that these can be identified and their quantities determined. The suitability of the column was tested by preparing and measuring real fermentation samples.
Conducting the test
The starchy raw materials wheat and corn and the sugary raw materials sugar cane and grapes were fermented. The following procedure was used for raw materials that contain starch: Firstly, the dry corn and wheat grains were crushed to a fine powder in a sample mill. After this, 40 g of the respective powder were dissolved in 200 g of water. This mixture was placed in a flask, and approx. 0.2 g of alpha-amylase were added while stirring continually. To ensure optimum conditions for the enzyme, the mixture was heated to 70 °C and left to sit at this temperature for at least 3 hours. The temperature was then lowered to 55 °C, 0.2 g of glucoamylase were added and also left for at least 3 hours. After this step, all samples were treated in the same way. Both the crushed sugar cane and grape extracts and the previously treated wheat and corn digests were mixed with 0.5 g of yeast and left to ferment at room temperature.
During the entire process, samples were taken from the digests at regular intervals. These samples were first centrifuged to separate the solid components. Next, a portion of the supernatant was filtered through a syringe pre-filter (0.45 µm). If the samples could not be measured immediately, they were stored at –20 °C to interrupt the fermentation process.
The samples prepared in this way were used directly for measurement under the analysis conditions listed in Table 1.
The retention times of the peaks were assigned by measuring individual standards, and calibration curves were created by measuring different concentrations.
| Analyseparameter | |
System |
|
Column |
Shim-pack SCR-102H |
Mobile phase |
water + |
Column oven |
50 °C |
Run time |
25 min |
Flow rate |
0.65 mL/min |
Injection volume |
10 μL |
Detection |
|
RID |
RID-20A |
Cell temperature |
50 °C |
PDA |
LC-2040 PDA |
Cell temperature |
50 °C |
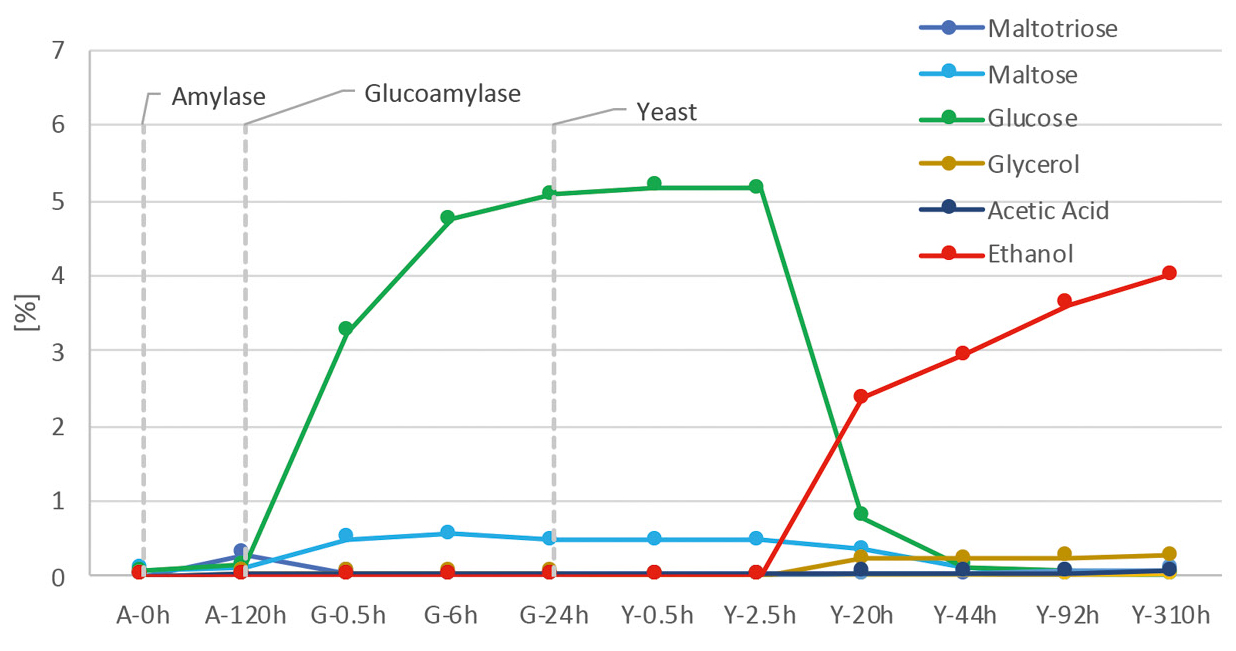
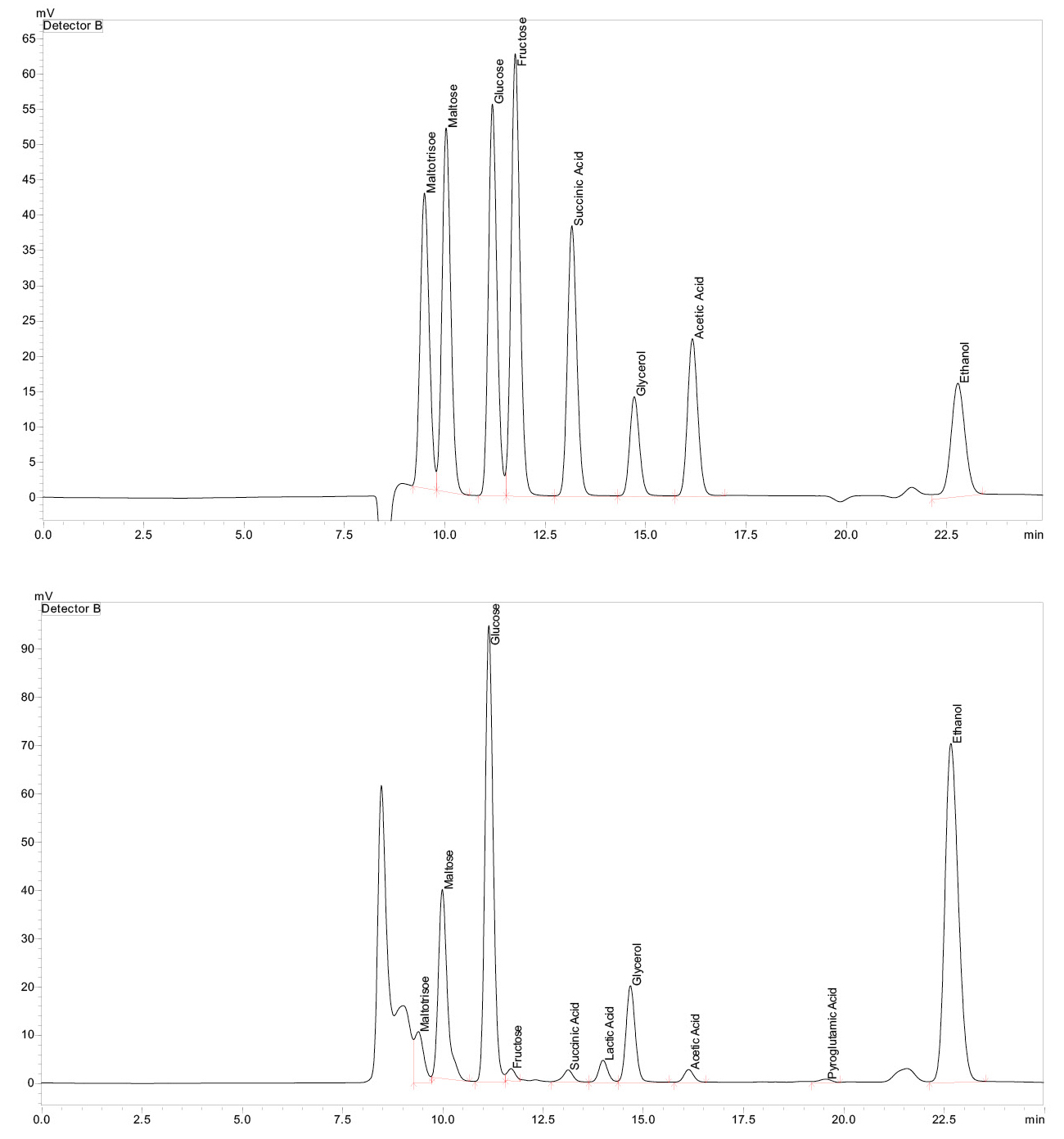
The data obtained in this way can be used to visualize the entire fermentation process, as shown in Figure 3 for the starting material corn. The figure shows that the sugars (maltotriose and maltose) can only be identified after alpha-amylase is added. This is followed by a sharp increase in glucose after glucoamylase is added. Ethanol is only produced after the yeast has been active for some time. Furthermore, it is clearly visible that the decrease in glucose is associated with a simultaneous increase in ethanol content.
While the initially formed sugars are completely broken down, ethanol becomes the main product after some time. The glycerine and acetic acid content also increase with the ethanol. Glycerine is a by-product of alcoholic fermentation and is produced by yeast. Glycerin contributes, among other things, to the viscosity and consistency of fermentation products like wine.[2] Acetic acid is produced by the universally occurring Acetobacter, which oxidize ethanol to acetic acid.[3]
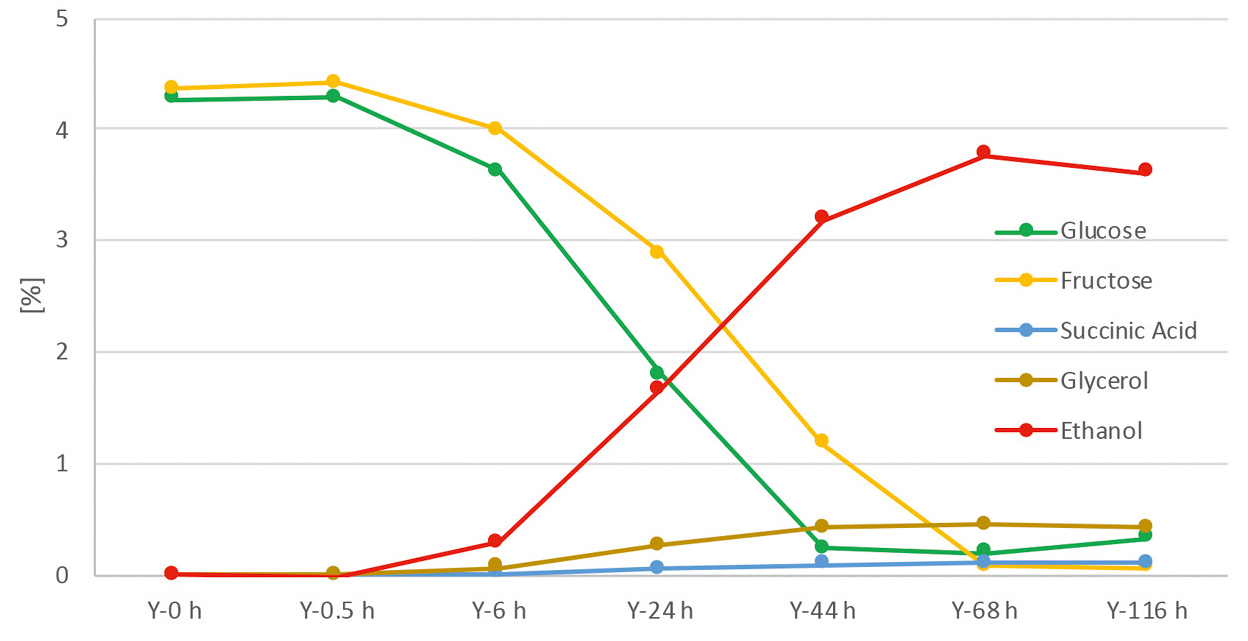
In contrast, sugary substances do not require the addition of amylase or glucoamylase, as the sugar they contain can be converted directly by the yeast. Figure 4 depicts the fermentation of grapes. Grapes contain roughly equal amounts of glucose and fructose. However, the yeasts favor the glucose, which is why it is broken down more quickly.
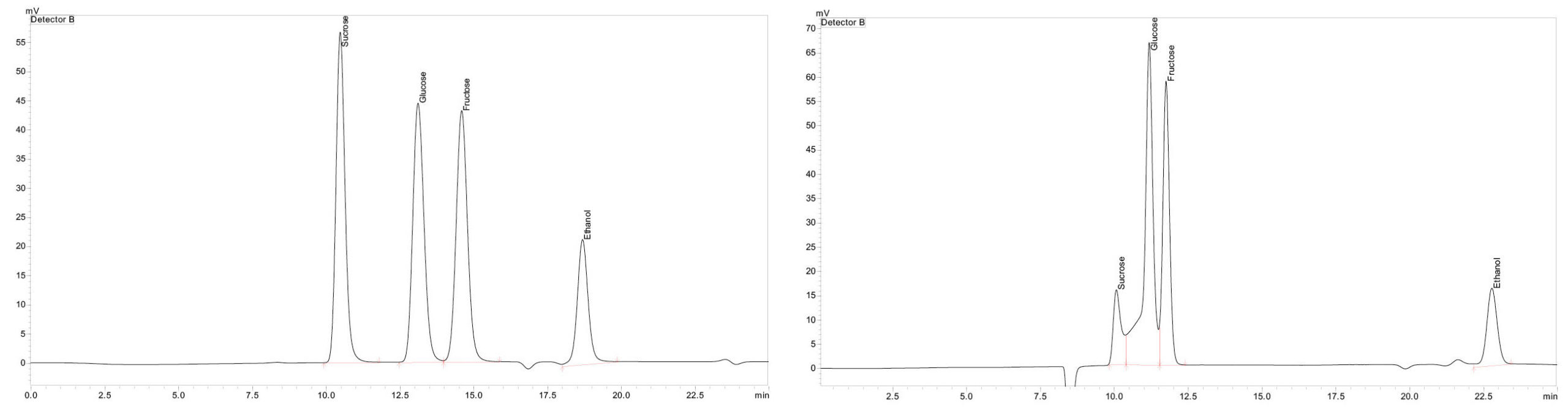
Other interesting raw materials are sugar cane and sugar beet, with sucrose being the predominant sugar in these plants. Sucrose, also known as granulated sugar or table sugar, is a disaccharide in which glucose and fructose are combined, although this bond is cleaved in an acidic environment. That means a column that works with an acidic mobile phase, as is the case with the SCR-102H, cannot be used for analyzing materials containing sucrose. For this reason, the carbohydrate-specific column Shim-pack SCR-101N, which can separate sugar in pure water, was used additionally. Figure 5 depicts the comparison of the sucrose, glucose, fructose and ethanol standards on the two columns. The decomposition of the sucrose can be clearly recgonized when using the acidic mobile phase, while the SCR-101N shows clear peaks.