Precision beats estimation – GFR measurement by iohexol plasma clearance
A ready-to-use solution for kidney function measurement
Shahriar Kermanshahian, Paul Gentil, Nephrolyx GmbH
The glomerular filtration rate (GFR) plays a vital role in monitoring the kidney function. Even though kidney diseases rank as the 10th leading global cause of death, resulting in 1.3 million deaths annually (2019), the vast majority of GFR determinations are based on estimation (eGFR). These estimations lack clinical accuracy, which can lead to uncertainty in clinical decision-making, especially for critical patient cases. According to the international Kidney Disease: Improving Global Outcomes (KDIGO) organization, reliable and accurate results can only be achieved by direct measurement of the GFR (mGFR) using exogenous markers such as iohexol.
Why life should be in the hands of precision
Imagine a life without precision and the ability to measure. Imagine if your car had no speedometer and you always had to estimate your speed. Or if the length of the jumps in the Olympic Games was estimated instead of precisely measured. Or if soccer referees had a watch but simply guessed when it was time for the final whistle. Sounds absurd? Well, in the vital field of kidney health, people have to actually estimate how badly a diseased kidney is affected or how to determine the right dosage of cancer treatment and stem cell therapy. The problems of this “guessing” dilemma of the glomerular filtration rate (GFR) are described below in detail – and the solution for a far better diagnostic method follows en suite.
The definition of the Glomerular Filtration Rate (GFR) and the dilemma of inaccuracy
Clinical assessment of kidney function is extremely important to the practice of medicine, and the glomerular filtration rate (GFR) is widely accepted as the best index of kidney function. Currently, estimated GFR (eGFR) based on serum creatinine or filtration markers such as cystatin C is widely used by clinical laboratories.[1, 2]
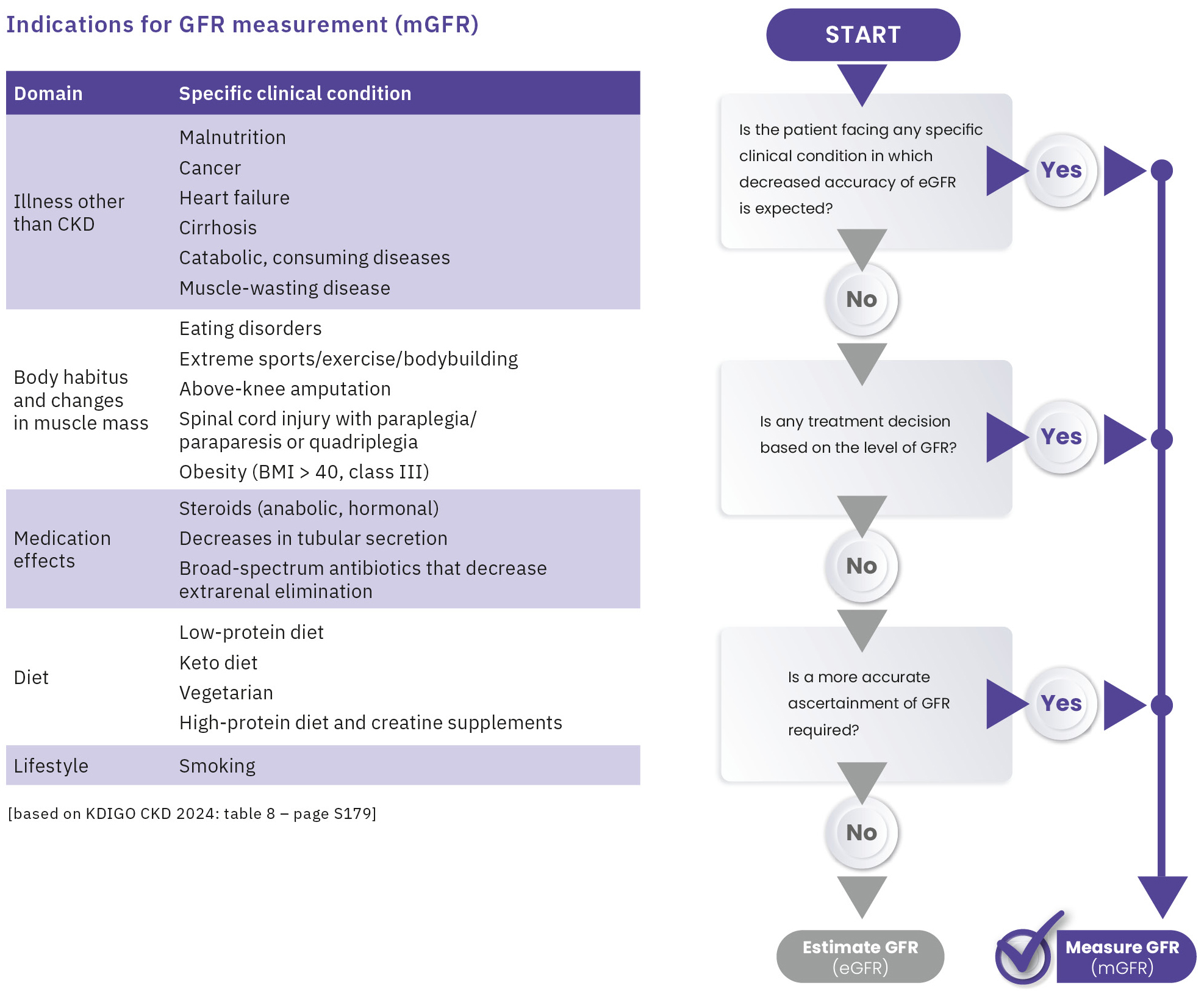
However, approximately 10–20 % of estimated kidney function values are deviating by more than 30 % from the measured GFR.[3] The estimated values are highly prone to error in many clinical settings such as patients with extremes of age and body size, severe malnutrition or obesity, diabetes mellitus, cancer, disease of skeletal muscle, paraplegia or quadriplegia and vegetarian diet. In these cases and circumstances such as evaluation for kidney donation or before administration of prolonged courses of nephrotoxic medications e.g., cytostatic agents or antibiotics, decisions based on inaccurate estimates may have harmful consequences.[2]
The solution: Measuring GFR using exogenous markers
Measuring GFR (mGFR) using plasma clearance of the exogenous marker iohexol is recognized as the gold standard by the latest KDIGO 2024 CKD Guideline.[4] The KDIGO Guideline identifies clinical scenarios where estimated GFR is prone to error and outlines selection criteria for patients requiring GFR measurement (Figure 1). The guideline also recommends that all nephrologists should ideally have access to at least one method to measure GFR using exogenous markers. Even though various methods for measuring GFR were available, they often required complex and time-consuming procedures, hence could not be easily performed routinely, until now.
Is there a method to establish easy and routine GFR measurement in laboratories?
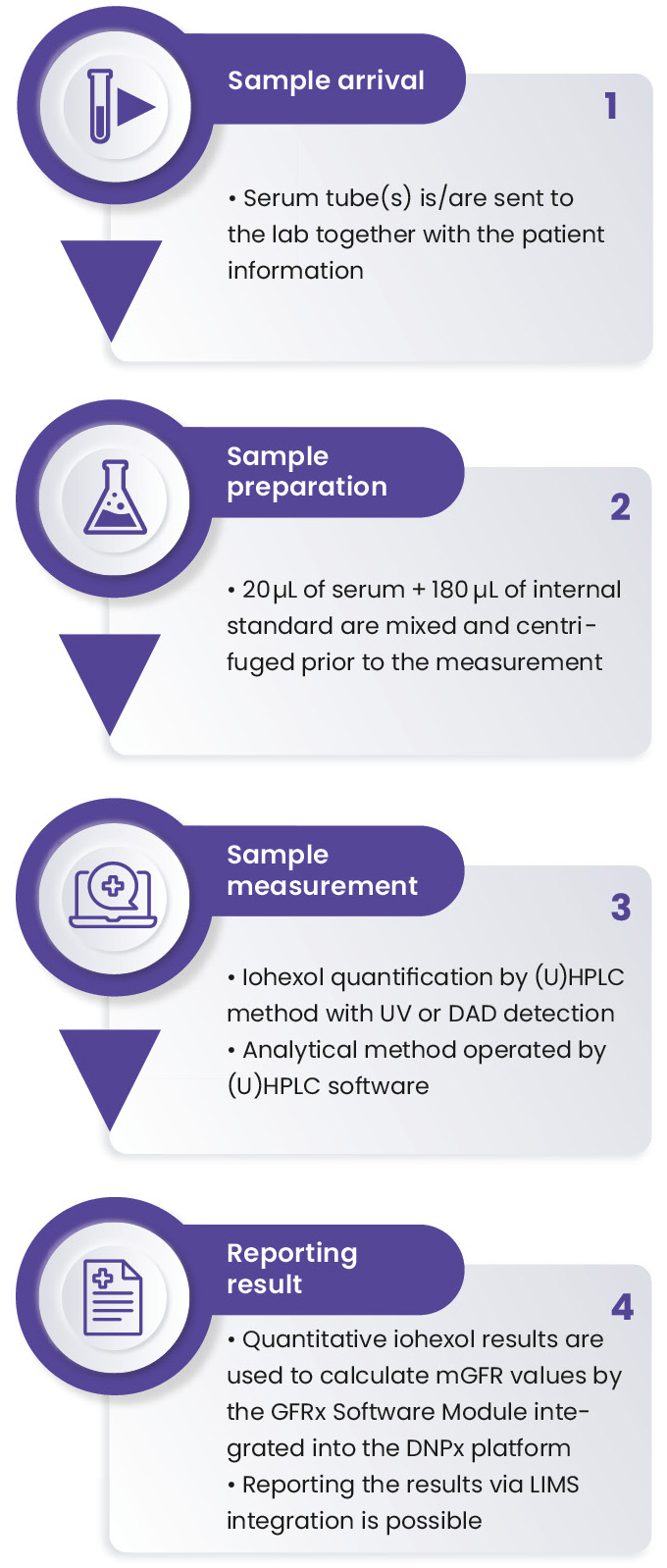
In order to answer this crucial demand, Nephrolyx has developed the CE-marked Nephrolyx IVDx kit and an associated software platform for GFR measurement via iohexol plasma clearance (Figure 2). The test is intended for the in-vitro diagnostic use to quantify the serum iohexol concentration, followed by the calculation of the resulting glomerular filtration rate (GFR) as an aid in the diagnosis, monitoring and treatment of kidney diseases. The main steps for the test are illustrated in Figure 3. This article describes the reliable protocol to determine iohexol concentration in human serum using the IVDx kit developed by Nephrolyx in a rapid and highly accurate measurement.
Material and methods
Sample preparation
The laboratory received human serum samples, collected after 3, 4 and 5 hours following a 5 mL iohexol injection to patients. To start sample preparation, 20 μL of each calibrant (C1−C6), quality control material (QC1−QC3) and sample were pipetted into the 96-well plate in duplicate. 180 μL of the Nephrolyx internal standard (IS) were added to each well. After 2 minutes of shaking at 1,000 rpm, the 96-well plate was set on the top of the capture plate and centrifuged for 30 minutes at 1,500 G. The filtrated samples were collected into the capture plate and ready to be measured on the (U)HPLC instrument.
UHPLC analysis
In this assay, the analysis was performed on ChromasterUltra Rs UHPLC (Hitachi) as reference and Nexera X3 UHPLC (Shimadzu) instruments. The separation of analytes on both systems was achieved via a binary gradient (details in Table 1) and an analytical column of Nephrolyx at +50 °C. The analytes were quantified using a UV-Vis detector.
Results
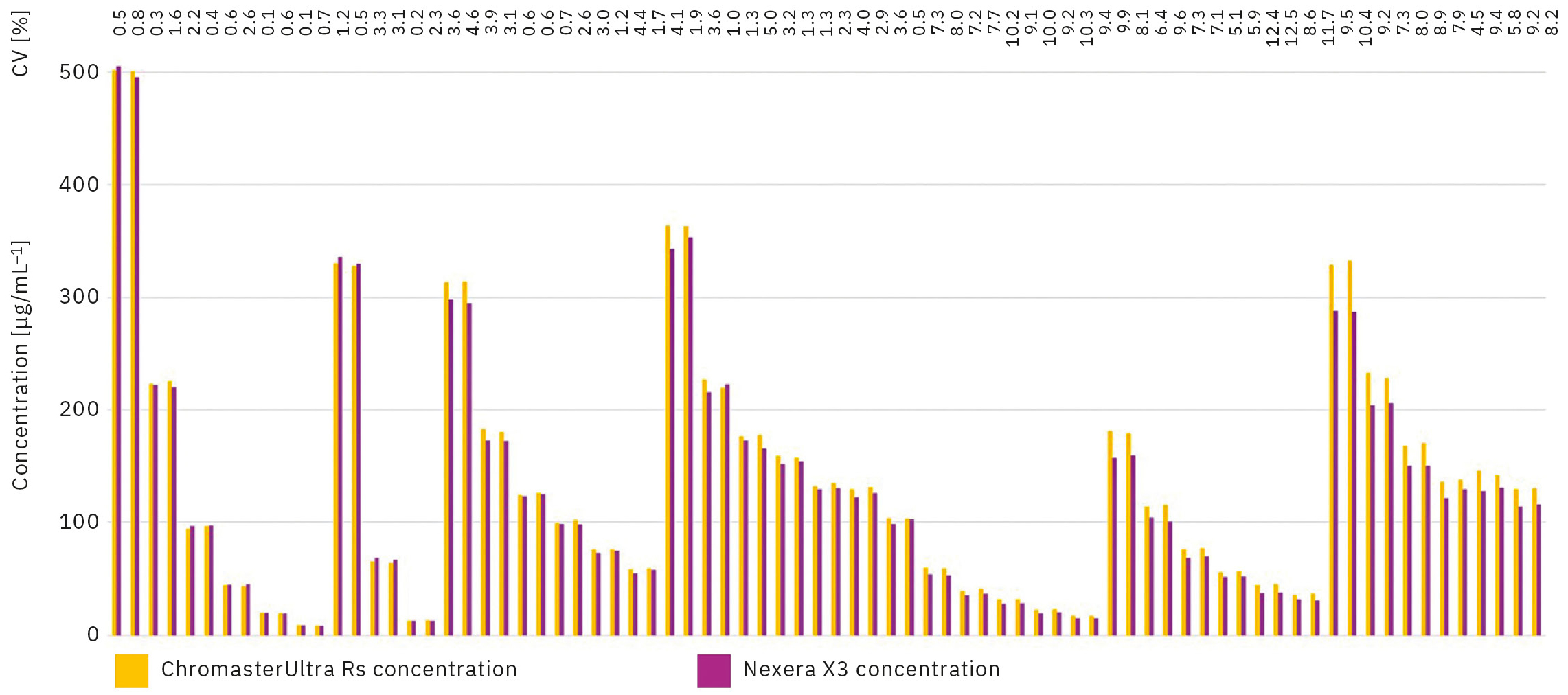
The results (Table 2) show very good reproducibility between the duplicates and high accuracy compared to the theoretical concentrations for both devices. The calculated values for the standard curves and the intra-/inter-batch deviations are shown in Table 3 and Table 4, respectively. For this assay, 50 samples were used to measure intra- and inter-assay precision. The intra-assay results show the standard deviation and bias calculated from the mean of the coefficient of variation (CV) between each duplicate. For the inter-assay precision, the accuracy and deviation of the obtained results for patient sample on the Hitachi ChromasterUltra Rs referenced to the Shimadzu Nexera X3 are calculated (Table 4).
The test replicated with the two instruments shows coefficients of variation in a range of 0.1 % and 12.5 % (Figure 4). These results are acceptable to conclude on the reproducibility of the Nephrolyx IVDx between the ChromasterUltra Rs and the Nexera X3.
| Time | Pump Settings | ||
| % A | % B | Flow | |
0.00 min |
100 % |
0 % |
1.0 mL/min |
1.00 min |
86 % |
14 % |
|
1.01 min |
0 % |
100 % |
|
1.05 min |
0 % |
100 % |
|
1.06 min |
100 % |
0 % |
|
2.50 min |
100 % |
0 % |
|
| Instrument | Slope | Intercept | R2 |
| ChromasterUltra Rs (Hitachi) | 187.7538 | 0.2199 | 0.9996 |
| Nexera X3 (Shimadzu) | 187.3024 | 0.2801 | 0.9995 |
| Sample Name | Theoretical concentration [μg/ml] | Average Measured concentration on ChromasterUltra Rs [μg/ml] | CV [%] between duplicates (ChromasterUltra Rs) | Deviation [%] (ChromasterUltra Rs to Assigned Value) | Average Measured concentration on Nexera X3 [μg/ml] | CV [%] between duplicates (Nexera X3) | Deviation [%] (ChromasterUltra Rs to Assigned Value) |
| C1 | 500.00 | 501.48 | 0.1 % | 0.3 % | 500.56 | 1.4 % | -0.1 % |
| C2 | 222.22 | 224.12 | 0.6 % | 0.9 % | 221.13 | 0.7 % | -0.5 % |
| C3 | 98.77 | 95.26 | 2.1 % | -3.6 % | 97.00 | 0.3 % | -1.8 % |
| C4 | 43.90 | 43.67 | 0.9 % | -0.5 % | 44.66 | 1.1 % | 1.7 % |
| C5 | 19.51 | 19.56 | 1.6 % | -0.2 % | 19.66 | 1.1 % | -0.8 % |
| C6 | 8.67 | 8.67 | 3.3 % | 0.0 % | 8.63 | 3.9 % | -0.5 % |
| QS1 | 333.33 | 329.03 | 0.5 % | -1.3 % | 333.06 | 1.2 % | -0.1 % |
| QS2 | 65.84 | 64.64 | 1.5 % | -1.8 % | 67.59 | 1.7 % | 2.7 % |
| QS3 | 13.01 | 13.08 | 1.7 % | 0.5 % | 12.85 | 0.5 % | -1.2 % |
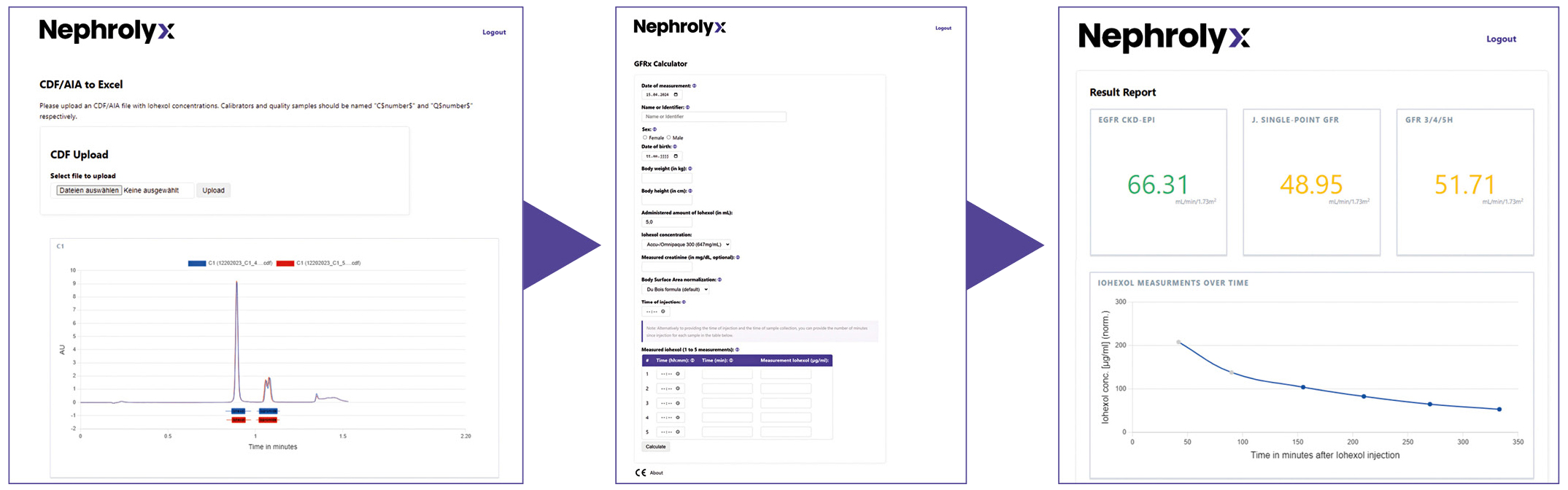
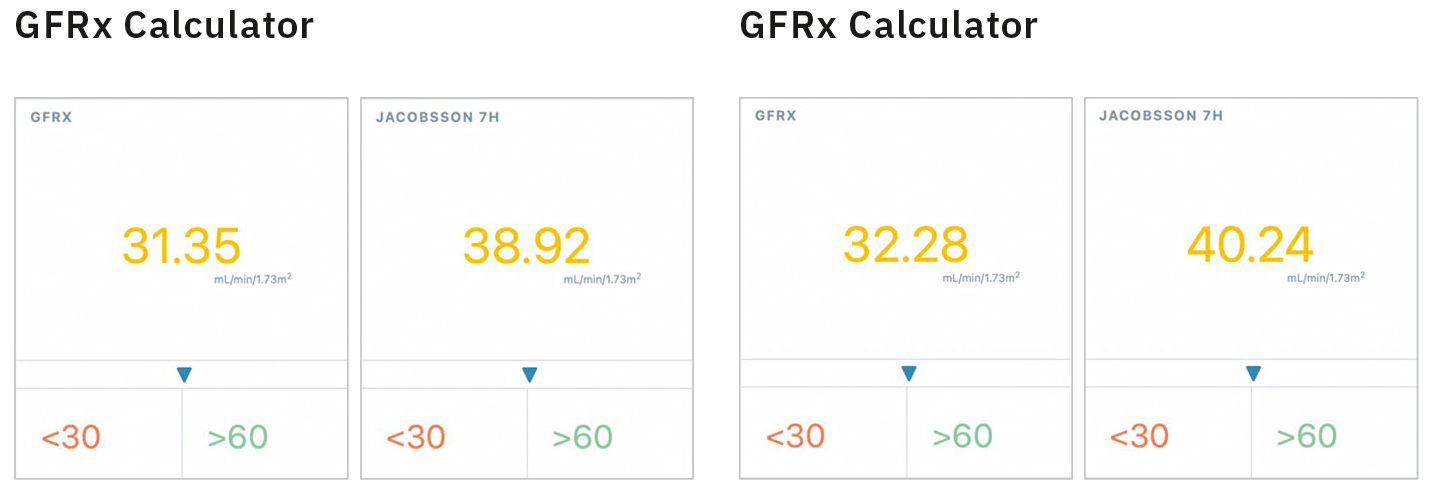
GFRx calculation
The GFR is calculated using the GFRx Software Module integrated into the Digital Nephrology Platform DNPx, developed by Nephrolyx (Figure 5). The patient sample presented in Table 4 was used to calculate the mGFR with the iohexol concentrations obtained from the ChromasterUltra Rs and the Nexera X3 (Figure 6). Deviations <= 3 % in the GFRx (multiple sampling point) and Jacobson (single sampling point) calculations, using the concentrations obtained from two instruments, are observed. Clinically, such a deviation between results is of low relevance.
| Sample Name | Average Measured concentration on Chromaster Ultra Rs [μg/ml] | CV [%] between duplicates (Chromaster Ultra Rs) | Average Measured concentration on Nexera X3 [μg/ml] | CV [%] between duplicates (Nexera X3) | Accuracy [%] (Chromaster Ultra Rs to Nexera X3) | Deviation [%] (Chromaster Ultra Rs to Nexera X3) |
| Patient T0 | 313.84 | 0.1 % | 296.28 | 0.9 % | 105.9 % | 5.9 % |
| Patient T1 | 181.40 | 1.0 % | 172.54 | 0.2 % | 105.1 % | 5.1 % |
| Patient T2 | 124.94 | 0.9 % | 123.90 | 1.0 % | 100.8 % | 0.8 % |
| Patient T3 | 100.58 | 1.6 % | 98.19 | 0.3 % | 102.4 % | 2.4 % |
| Patient T4 | 75.83 | 0.1 % | 73.59 | 1.9 % | 103.0 % | 3.0 % |
| Patient T5 | 58.61 | 1.1 % | 56.17 | 3.7 % | 104.3 % | 4.3 % |
Mastering accuracy and abolishing deaths related to kidney disease
After a direct comparison of the standard curves created on the ChromasterUltra Rs (Hitachi) and the Nexera X3 (Shimadzu), the calibrants and QCs measurement and the well-to-well comparison, it can be concluded that the Nephrolyx IVDx kit showed highly accurate and robust results on both devices.
Additionally, the Nephrolyx IVDx demonstrates that a CE-compliant solution can be established in laboratories to deliver fast and accurate results, enabling GFR measurements in daily clinical practice according to the new KDIGO CKD 2024 Guideline.
The vision is to provide hospitals and universities worldwide with this new method and make the number of 1.3 million kidney disease related deaths annually a thing of the past as well as guesswork and poorly-dosed medication that causes stress to already exhausted patients.
[1] Miller W.G. (2008). Am J Kidney Dis 52: 645–648.
[2] Stevens L.A., Levey A.S. (2009). J Am Soc Nephrol. 20: 2305–2313.
[3] Levey A.S., Becker C., Inker L.A. (2015). Jama. 313: 837–846.
[4] KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. (2024). 105 (4S): S117–S314.