Metal-free for better results – inert LC columns
New solution for the analysis of critical components
Dr. Martin Meyer, Shimadzu Europa GmbH
In recent years, increasing demands in the analysis of biological material, particularly in medical research, have resulted in more and more liquid chromatography columns (LC columns) being marketed as bioinert. The main objective is to reduce the metal interaction between the target molecule and the column hardware. This should lead to better sensitivity and reproducibility. This article describes the principle of adsorption, how inert columns can prevent it and which solutions Shimadzu offers.
A large number of LC-column providers who have included inert columns in their portfolios advertise that there are no interactions between the metal of the column and the analytes and that the results of analysis are therefore more precise. In comparison, in standard stainless steel columns interactions between column metal and analytes are possible. Depending on the analytes, the difference between a standard and a bioinert column can be considerable (Figure 1).
The processes that lead to adsorption effects on the metal of the column can be differentiated into two phenomena. On the one hand, the release of metal ions from the column or the system, which then accumulate, for example, in the stationary phase can lead to ionic interactions there. The second effect is the coordinated action of the positive metal surface on electron-rich analytes. Both effects result in a portion of certain analytes remaining on the column longer or not breaking away from the column at all. This results in losses of sensitivity or a degradation of peak form.
Molecules that carry phosphate groups, such as oligonucleotides and phosphopeptides, are affected. However, tetracyclines and mycotoxins and other molecules can also suffer significantly from the consequences of metal interaction.[1, 2] Furthermore, proteins and peptides can be susceptible to metal ion catalyzed decomposition, which is also prevented by the use of bioinert columns.[3]

|
Method |
Disadvantage |
|
Surface passivation with acids |
Temporary |
|
Surface passivation with sample or matrix |
Not stable |
|
Chelating agent |
Ion suppression |
|
Other metals such as titanium and MP35N (a nickel-cobalt alloy) |
Interaction/washout of metals still possible |
|
Pure PEEK columns |
Low pressure stability |
|
Glass-covered |
Frit made of another material -> PEEK or coating |
|
PEEK-lined |
Limited solvent compatibility |
|
Coatings |
– |
The effects of metals on chromatography have been known for more than 20 years.[4] To date, various methods have been applied to solve the problems of metal interactions (Table 1).
There are a range of temporary solutions such as passivation with acid or with the sample itself, but these passivations must be renewed after some time and make the method itself less robust. Alternative solutions, such as the use of metals other than steel, can reduce some of the adsorption effects, but ultimately metal is still used, which can still cause interactions.
The two most commonly used techniques by inert column manufacturers are lining the metal column with a polyetheretherketone (PEEK) polymer layer and coating the metal surface of the column. Shimadzu offers both variants: the coated columns under the name Shim-pack Scepter Claris (“CL” for short) and the PEEK-lined columns with the addition “metal-free” or “MF” for short. The advantage of this combination is that the columns do not interact with metal-sensitive analytes but still offer the high-pressure stability of typical metal columns for liquid chromatography.
Effects of inert components
The phenomena that lead to adsorption are shown in Figure 2 as well as the use of inert column material to prevent interactions. To investigate the effects of these inert components, the applicability of the inert Shimadzu Nexera XS system and the metal-free Shim-pack Scepter columns for the analysis of nucleotides and phosphopeptides was tested. Both substances are relevant for medical research. For example, oligonucleotides are important for the treatment of rare diseases [5], among other applications, and phosphopeptides can be used to obtain new findings in cancer research.
The detailed analysis conditions for nucleotides and phosphopeptides are listed in Table 2. For sample preparation no standard glass vials are to be used to store samples because these also have metals on the glass surface and adsorptions can occur. Therefore, the use of vials with low adsorption such as TORAST-H Vials from Shimadzu is recommended. The Nexera XS inert system, in which the entire flow path is inertly lined, was used for all analyses. As a result, the system is ideally coordinated for the inert column hardware.
|
Analyte |
Nucleotides |
Phosphopeptides |
||||||||||||||||
|
System |
Nexera XS inert |
Nexera XS inert |
||||||||||||||||
|
Column |
Shim-pack Scepter C18 (metal-free) |
Shim-pack Scepter C18 |
||||||||||||||||
|
Mobile phase |
Acetonitrile/water + 50 mmol ammonium formate = 0.5:99.5 |
A) Water + 0.1 % formic acid |
||||||||||||||||
|
Gradient |
Isocratic |
B conc. 5 % (0 min) → 50 % (12–13 min) → 5 % (13.01–16 min) |
||||||||||||||||
|
Flow rate |
0.2 mL/min |
0.3 mL/min |
||||||||||||||||
|
Injection volume |
2 µL |
1 µL |
||||||||||||||||
|
Oven temperature |
40 °C |
40 °C |
||||||||||||||||
|
Sample |
Equimolar (50 µg/mL) mixture of adenosine triphosphate, adenosine diphosphate and adenosine monophosphate, dissolved in pure water |
MS PhosphoMix 2 Light (40 nmol/L) |
||||||||||||||||
|
Vial |
TORAST-H Vial (low-adsorption glass vials) |
TORAST-H Bio Vial (PP vials) |
||||||||||||||||
|
Detector |
PDA (254 nm) |
LCMS-8045 |
||||||||||||||||
|
Detector settings |
PDA cell: Standard (inert for Nexera XSi) |
|
Results
Nucleotides
The adsorption of metals can be recognized by a poor recovery and a strong peak broadening of the adsorbed components due to an intensified interaction. In fact, the peak forms and the sensitivity of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are poor if a conventional column is combined with a standard UHPLC system. The use of an inert column and an inert system provides the best results, with very symmetrical peak forms and the highest signal intensity (Figure 1).
When working with conventional columns and systems, an especially important factor is the influence of the injection number on sensitivity and peak form. The reason for this is that the analyte forms a passivation layer on the metal surface as the number of injections increases. This is illustrated in Figure 3, in which the tailing factor serves as a function of the number of injections. Values that fluctuate greatly over the course of the injections indicate poor reproducibility. Some users intentionally utilize the passivation effect of the sample or other substances to condition their system, but this passivation layer is not permanent and dissipates over time. Therefore, the completely inert configuration offers the best conditions for the analyzed nucleotides.

Phosphopeptides
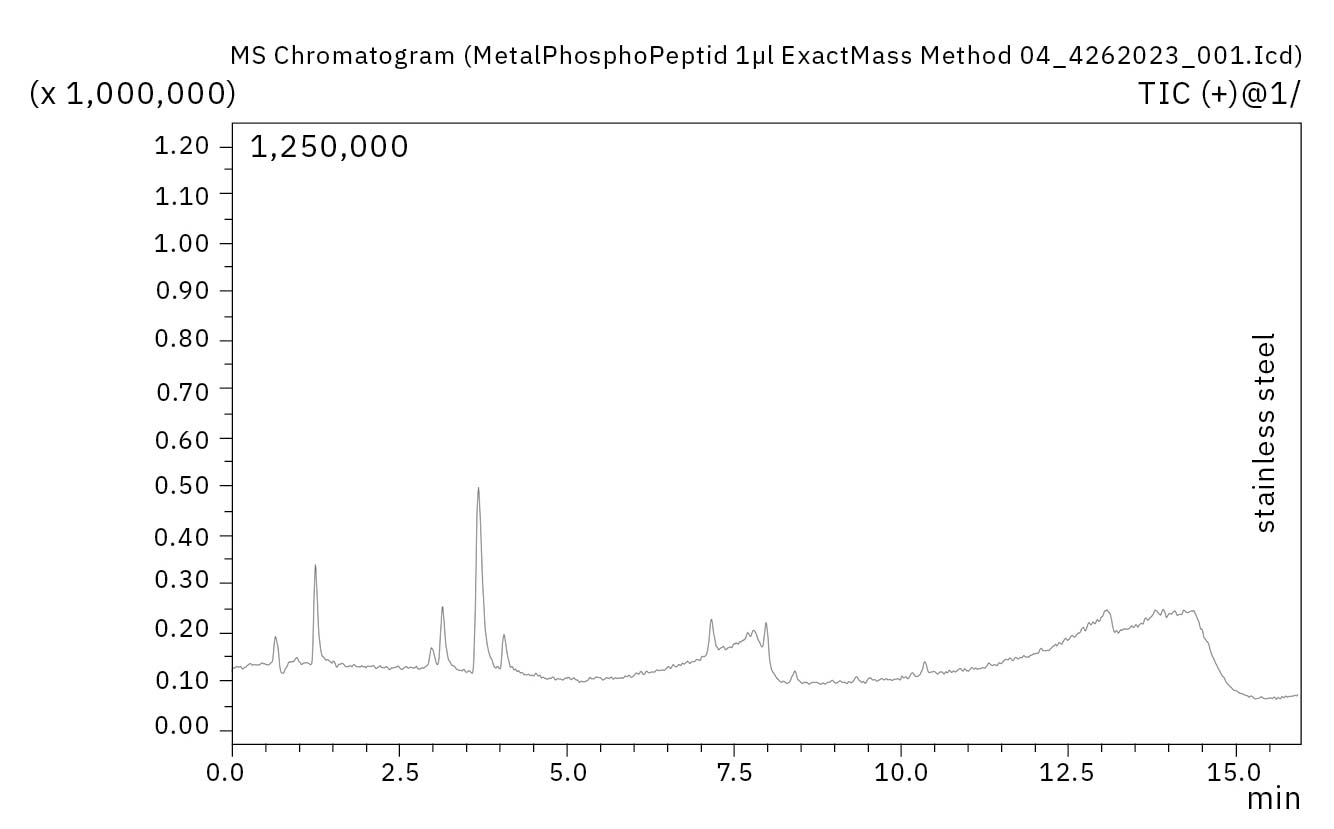
The standard column with metal material performed poorly in the analysis of the phosphopeptide mixture. Several compounds were not detectable, and the available compounds showed small, wide peaks. A considerable increase in performance was achieved by using the bioinert columns. Not only was it possible to detect all compounds present in the standard mixture, but this occurred with at least 50 % higher sensitivity compared to the non-inert column (Figure 4).
Furthermore, the quantity or position of the phosphate group influences the degree of adsorption. Thus, some compounds can also be detected on the metal column, while others are no longer detectable at all.
More sensitivity in the analysis
The use of metal-free components both in the LC device and in the LC column substantially improved the sensitivity of the nucleotide analysis. Additionally, the reproducibility is better compared to conventional systems. All phosphopeptide components could be detected with the coated columns and with PEEK-lined columns. Compared to this, when using a metal column, some compounds were adsorbed to such a great extent that they were no longer detectable. The sensitivity of both bioinert modified columns was similar, making Shimadzu columns a very good option for the analysis of phosphopeptides.
[1] D’Atri, V., Murisier, A., Fekete, S., Veuthey, J.L., Guillarme, D., Current and future trends in reversed-phase liquid chromatography-mass spectrometry of therapeutic proteins, TrAC Trends in Analytical Chemistry, Vol. 130, 2020.
[2] Anspach, J.A., Rao, S., Rivera, B., Bioinert versus biocompatible: the benefits of different column materials in liquid chromatography separations, LCGC North Am., Vol. 36, 2018, pp. 24–29.
[3] Zang, L., Carlage, T., Murphy, D., Frenkel, R., Bryngelson, P., Madsen, M., Lyubarskaya, Y., Residual metals cause variability in methionine oxidation measurements in protein pharmaceuticals using LC-UV/MS peptide mapping, Vol. 895–896, 2012, pp. 71–76.
[4] Euerby, M.R., Johnson, C.M., Rushin, I.D., Sakunthala Tennekoon, D.A.S., Investigations into the Epimerisation of Tipredane Ethylsulphoxide Diastereoisomers during Chromatographic Analysis on Reversed-Phase Silica II. The Involvement of Metals in Commercially Available C18 Silicas, J. Chromatogr., Vol. 705 (2), 1995, pp. 229–245.
[5] Schad, G., Erxleben B.T., Improving biopharmaceutical drug development, Secrets of Science 1/2023, https://www.shimadzu-webapp.eu/magazine/issue-2023-01_en/improving-biopharmaceutical-drug-development/.