Copper bottles: (un)healthy trend?
Determination of the concentration of the trace element copper with the Shimadzu AA-7800
Dr. Johannes Hesper, Shimadzu Europa
The use of copper vessels for storing and consuming water has a long tradition in some cultures. In recent years, copper bottles or cups have also gained popularity in our part of the world. In addition to the material’s antibacterial properties, manufacturers often mention the enrichment of the water with trace elements. Copper (Cu) is an essential trace element for the human body. The use of copper bottles causes small quantities of copper to be released into the water. The Shimadzu AA-7800 Atomic Absorption Spectrophotometer was used to analyze how large and how healthy these quantities actually are.
Copper bottles for drinking water are very trendy right now. Their use has increased in the past few years, and manufacturers often advertise their positive health effects, for example in the sense of Ayurveda. But how much of the essential trace element copper is actually released in a Cu bottle? And, in this case, does the drinking water still comply with the recommendations of the current German Drinking Water Ordinance?[1] A new version of this ordinance entered into force on June 24, 2023 and provides for the introduction of risk-based drinking water protection. The ordinance establishes lower limit values for harmful substances such as chromium, arsenic and lead, while the limit values for copper (Cu) remain unchanged. It prescribes a maximum value of 2 mg/L (2 ppm) of copper, which should not be exceeded.
To determine the quantity of the copper migration, an uncoated, cleaned copper bottle (99.7 % copper, manufacturer’s declared value) was filled with tap water and stored closed for six days at room temperature. On days zero, one, two and six, the copper content was determined using the new AA-7800 Atomic Absorption Spectrophotometer in flame mode (acetylene flame).
On day zero, three samples (blind samples) were taken from the bottle and acidified with 1 % HNO3 (nitric acid) to stabilize the copper in solution. On the next day, three more samples were drawn and acidified. The same procedure was repeated on day two and for a fourth and last time on the sixth day.
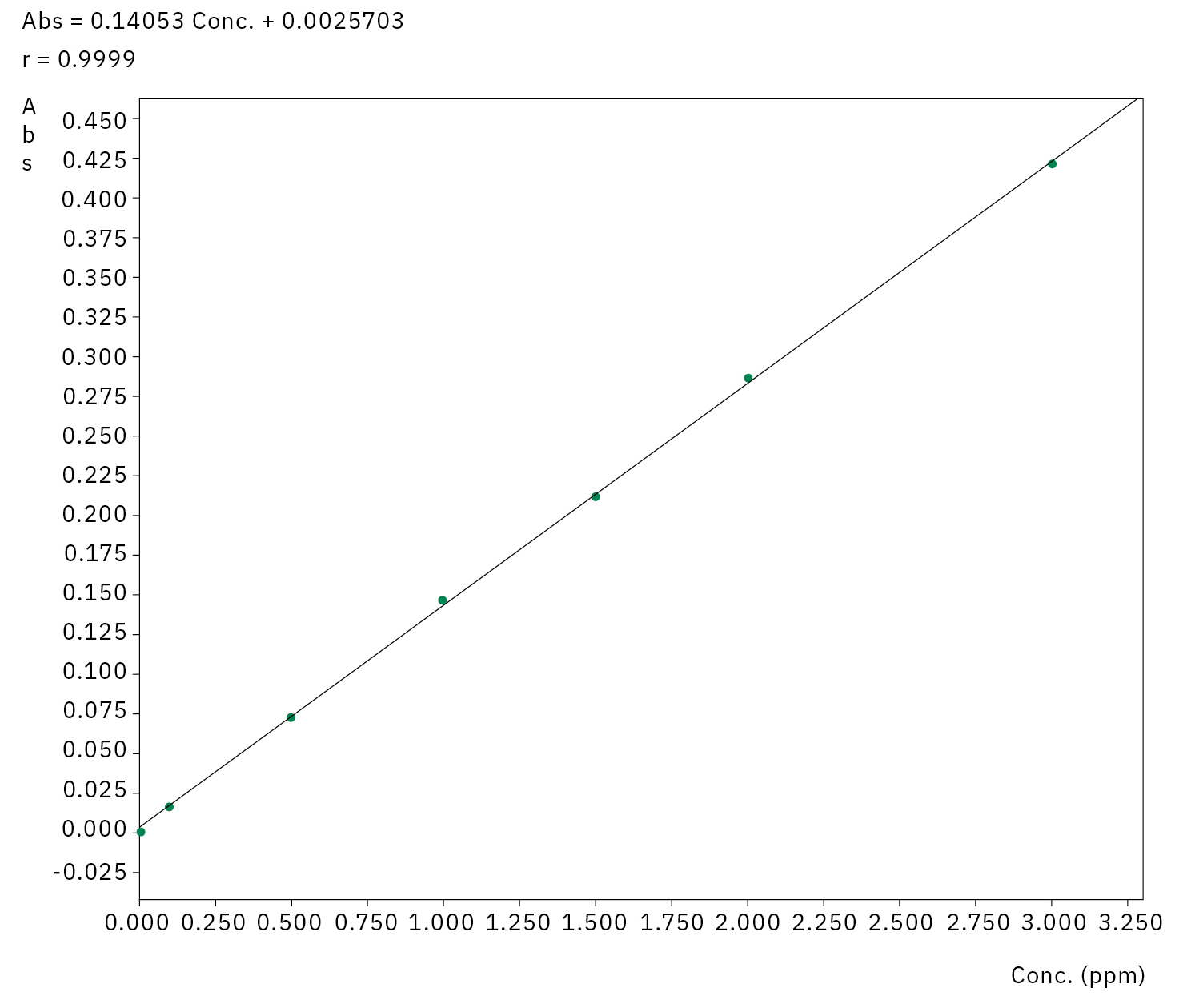
Finally, all the samples were measured against an external copper calibration. The calibration line obtained is illustrated in Figure 2 and shows a very high linearity in the measuring range of 0–3 ppm Cu with R2 = 0.9998.
The proven standard measurement settings for copper from the Shimadzu cookbook (Flame AAS) were used. Using the copper (Cu) hollow -cathode lamp, the copper absorption was measured at 324.8 nm in the acetylene flame and determined against the calibration.
The measurement results are listed in Table 1.
|
Day |
1 |
2 |
3 |
Mean |
SD |
0 |
0.0294 |
0.0265 |
0.0287 |
0.0 |
0.005 |
1 |
1.0405 |
1.0619 |
1.059 |
1.1 |
0.035 |
2 |
1.7692 |
1.7728 |
1.7934 |
1.8 |
0.039 |
6 |
3.6293 |
3.5389 |
3.8321 |
3.7 |
0.450 |
While the measured value on the sixth day, at 3.7 ppm, was a little bit outside the working range of 0–3 ppm, it can nevertheless be assumed that the calibration retains its validity until then and that the absorption signal remains linearly dependent on the Cu concentration.
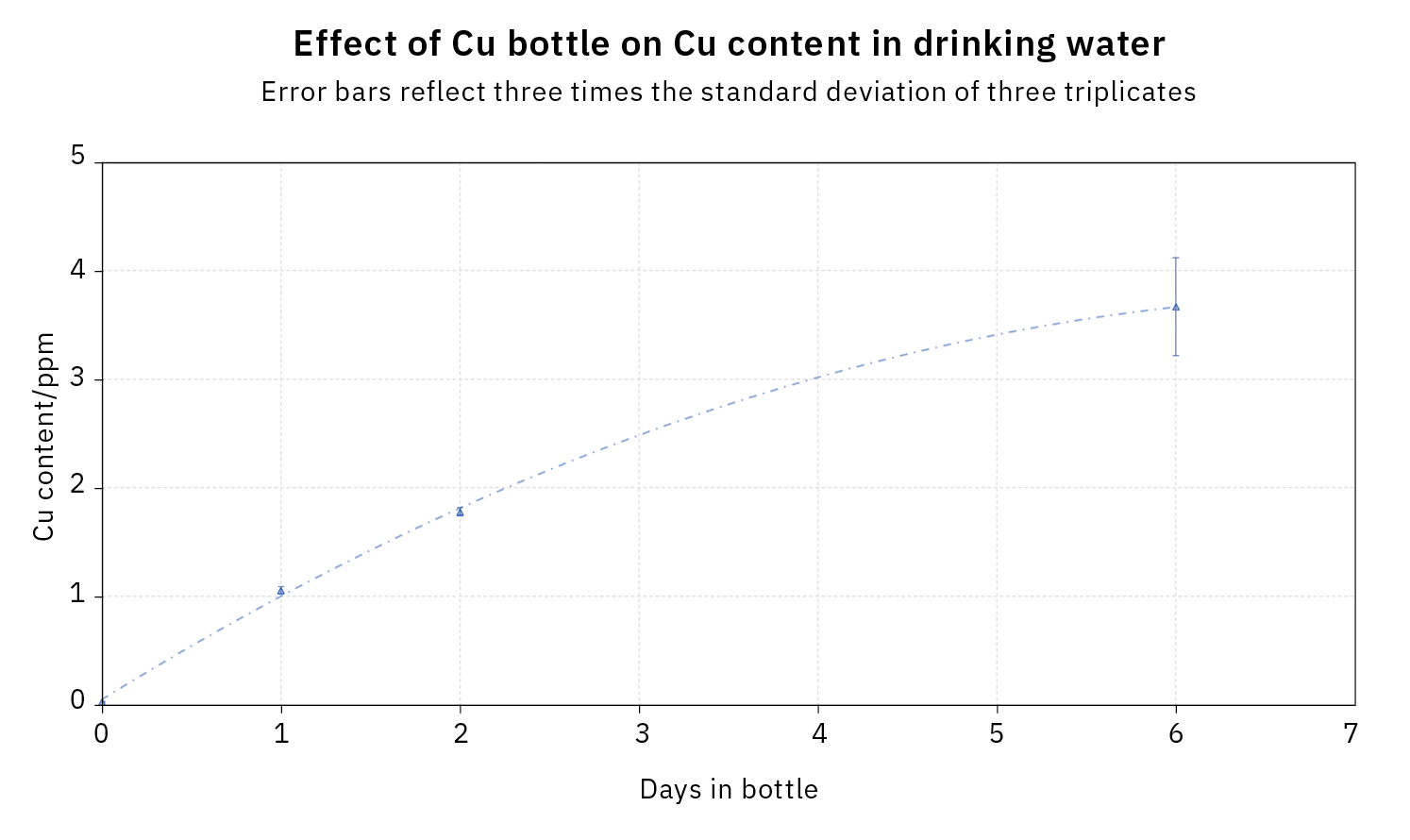
Therefore, the copper content of the drinking water increased daily from the original value of 0 ppm (approx. 30 ppb) to be above the limit value in the German Drinking Water Ordinance on the sixth day, at 3.6 ppm Cu. The limit value of 2 mg/L copper was probably already exceeded on the third day, but no samples were taken on this day.
The Shimadzu AA-7800 Atomic Absorption Spectrophotometer, with up to eight hollow-cathode lamps, offers two optimal background compensations for the correction of the sample matrix. The high-current pulse technology (SR method) and the deuterium (D2) background compensation are available as standard for measurement with flame and graphite tube. Thanks to the optional autosampler ASC-7800, up to 60 samples can be measured comfortably. The comprehensive safety functions also make the use of acetylene gas extremely safe, which makes it ideal for the analysis of trace elements even in difficult matrices.
Using copper bottles safely
Anyone who is considering using a copper bottle should make sure that it is made of high-quality copper in order to prevent possible health risks due to heavy metals. This is because copper can be toxic in large quantities and lead to copper poisoning. This expresses itself in symptoms including nausea, vomiting, abdominal pain and other health problems.
Based on the non-representative measuring results, drinking water should be stored in a copper bottle for a maximum of two days because otherwise the recommendations of the German Drinking Water Ordinance are exceeded. Drinks and fruit juices that contain acid should not be stored in a copper bottle because otherwise an accelerated release of copper is to be expected.
[1] TrinkwV, Verordnung über die Qualität von Wasser für den menschlichen Gebrauch (Trinkwasserverordnung – TrinkwV) [Ordinance on the Quality of Water for Human Consumption (Drinking Water Ordinance – TrinkwV)] of June 20, 2023 (BGBl. 2023 I No. 159, pg. 2) https://www.bundesgesundheitsministerium.de/service/begriffe-von-a-z/t/trinkwasser/neue-trinkwasserverordnung.html