An integrated systems approach to medicinal gas
Improving UV spectroscopy for analysis of medicinal nitric oxide mixtures
Riccardo Nava, Massimiliano Cattaneo, Simone Baccaro, SOL GROUP
The medical applications of nitric oxide (NO) are significant and growing. A recent collaboration between Shimadzu and the SOL Group – who produce both an active ingredient and a finished medicinal product based on nitric oxide – has led to a new system-integrated solution that streamlines analytic performance in the quality control of an NO-based medical gas. This article presents the results of that cooperation – which make the process of drug batch release faster, more accurate and more reliable – and hints at its potential to improve other analytical applications for gaseous samples.
Developing medical gases
The SOL Group, established in 1927, is a multinational company headquartered in Italy that focuses on the production and marketing of technical and medical gases. At its Specialty Gases Production Facility in Monza (near Milan), ultrapure gases and high-accuracy scientific mixtures are produced for customers such as research centers, universities, laboratories and the pharmaceutical industry. The Monza facility is authorized for the manufacture of gaseous active drug substances and drug products by the European Medicines Agency (EMA) – a decentralized agency of the European Union (EU) responsible for the scientific evaluation, supervision and safety monitoring of medicines in the EU – and thereby meets the criteria of their Good Manufacturing Practice (GMP).
It was in Monza that a new medicinal gas using nitric oxide as an active ingredient was developed and is today produced (Figure 1). That product is registered with a marketing authorization in Europe under the name Neophyr and is widely used in European hospitals. Indeed, the market demand for nitric-oxide-based medicinal products in general is growing, due to the increasing spread of therapeutic applications aiming to improve the health of patients.
Ensuring parts-per-million quality
In the production cycle of a gaseous drug mixture in which the active ingredient is diluted on a parts-per-million scale in an (inactive) excipient – such as pure nitrogen – it is vital to comply with all GMP requirements to avoid any risk of contamination of the final product. It is also essential at all stages to ensure the most stringent quality assurance as well as the highest production quality – for instance, by means of sophisticated mass comparators for the accurate dosing of components, advanced automation and traceability software.
In particular, to guarantee the final quality of each product, the SOL Laboratory in Monza (Figure 2) uses instruments that can estimate, with absolute precision, values of impurities in a parts-per-million order through the use of detection systems based on multiple techniques.
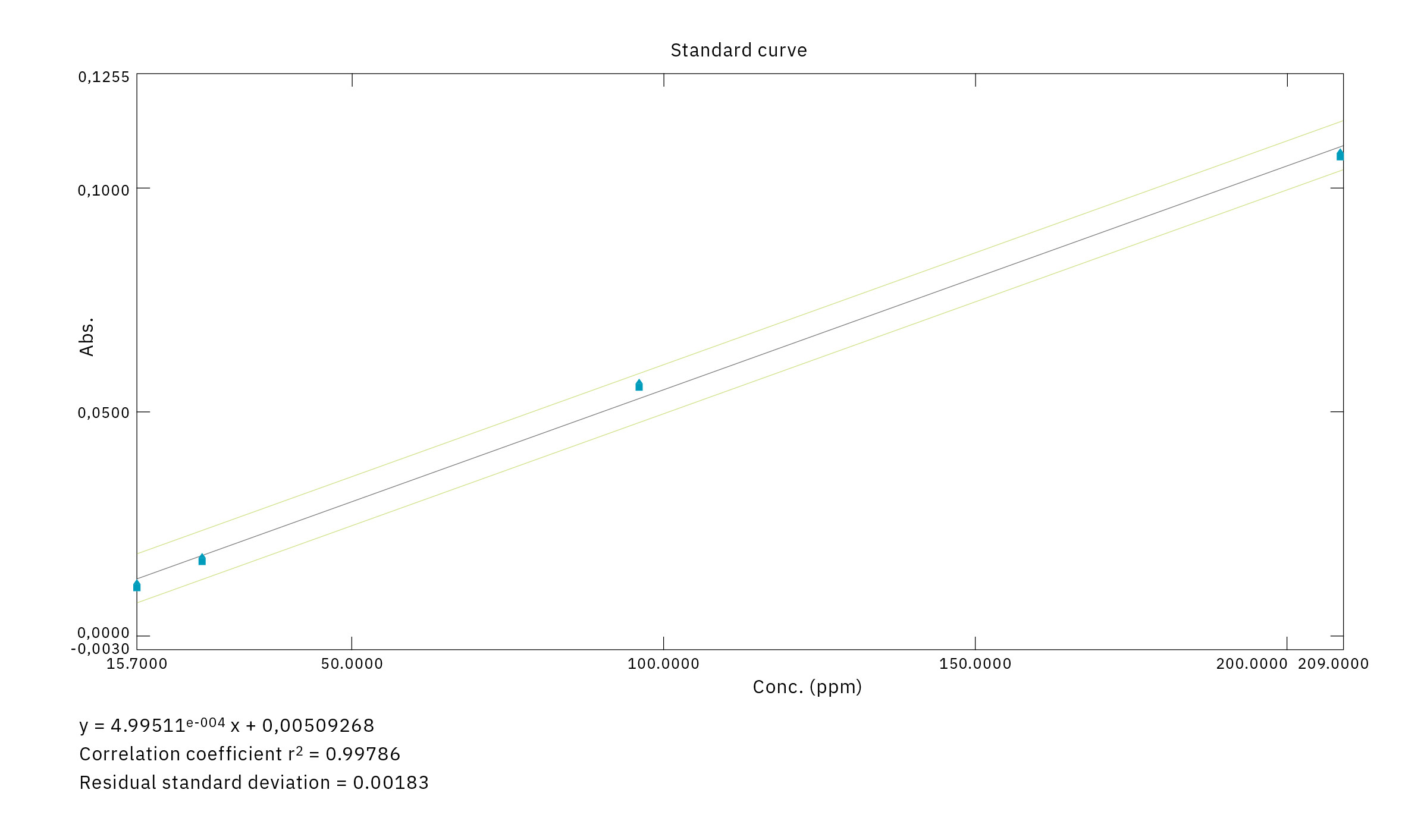
In order to obtain an active pharmaceutical ingredient and intermediate product in full compliance with GMP requirements – and especially regarding the Nitrogen Dioxide (NO2) impurity content – the Monza lab concluded that they needed to upgrade to the next level of technologically advanced instrumentation. Reference was made to the European Pharmacopoeia [Ph. EU monograph of the NO: EU Ph. 01/2008:1550], in which a UV-Vis spectrophotometer reading at a wavelength of 400 nm is specified as a reference technique for the analysis of Nitrogen Dioxide impurity.
Working toward a new SOLution
In view of the gaseous nature of the samples to be subjected to analysis and the need to have precise levels of accuracy for the various NO2 concentrations to be analyzed, the Monza lab needed an instrument equipped with a specific gas cell in which the optical path was several meters in length and which was capable of reflecting electromagnetic radiation a certain number of times thanks to a system of mirrors inside the cell.
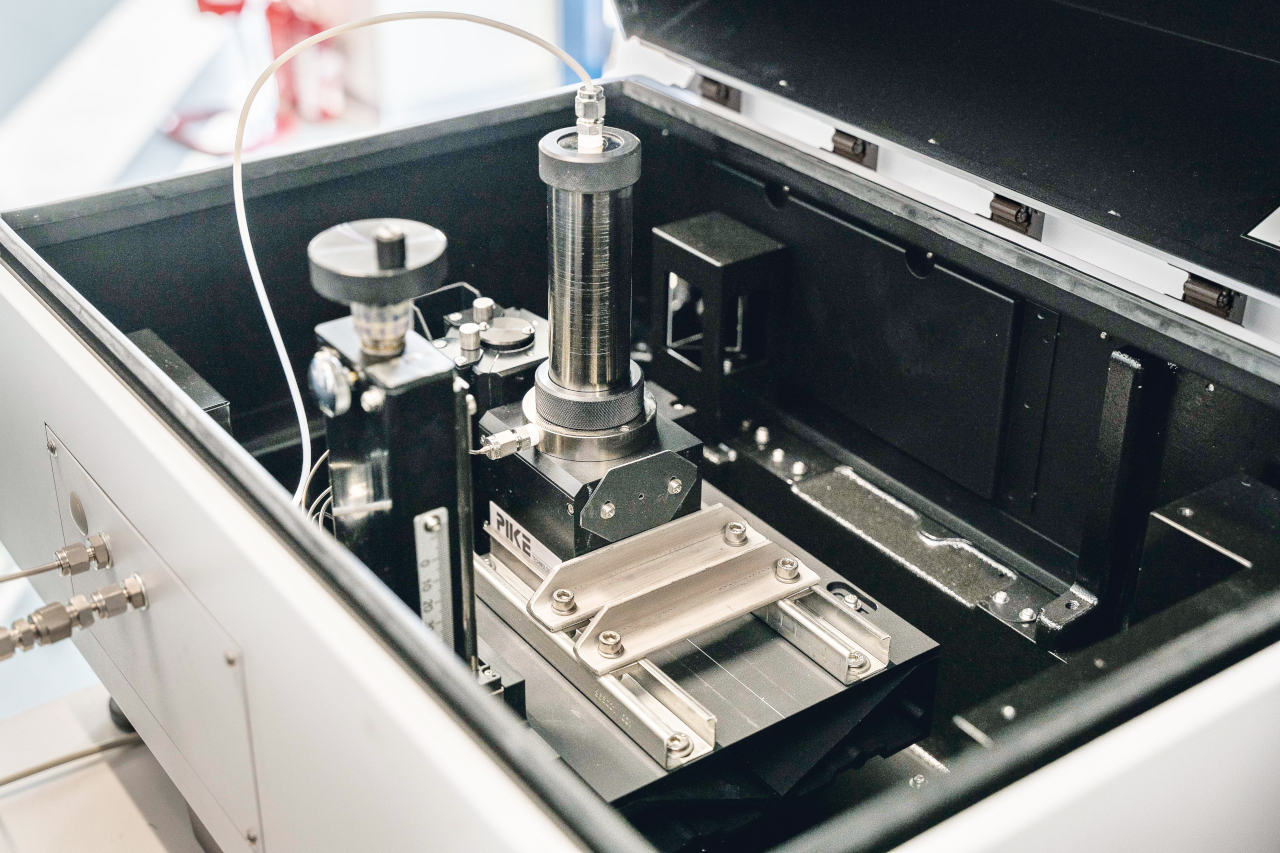
The SOL lab team at Monza identified one such instrument of special interest: Shimadzu’s UV-2700 spectrophotometer, in which the 2.4 m long PIKE gas cell could be installed with an MPC-2600 module. Just as interesting was Shimadzu’s interest in working with the lab to adapt and fully utilize the instrumentation for the lab’s specific needs.
Together with technical support personnel from Pentatec and Selas Lab (Figure 3), experts from Shimadzu Italia and the SOL Monza team worked closely to optimize the analysis system in terms of gas cell fastening and alignment. By employing a quartz window inside the cell and a specially designed sampling piping, the instrument was also made ready for use for the analysis of pressurized samples, thereby lowering the analytical detection threshold. The system integration solutions adopted (Figure 4) gave positive results on the analyses, especially in terms of measurement repeatability and instrumental detection limits.
This tailor-made solution also revealed potential enhancements that could be made to other analytical applications used on gaseous samples and for which analytical techniques such as gas chromatography or infrared spectrophotometry – but lacking the advantages of a system-integrated approach – are currently more common.
Collaboration to improve the state of the art
Today, the collaboration between SOL and Shimadzu is making the quality assurance process of an important drug even faster, more accurate and more reliable. This firmly supports the continued expansion of helpful medical applications of nitric oxide as well as offering an intriguing possibility for improving other analytical applications for gaseous samples.