Ethanol as a blending component for petrol
Determination of higher alcohols and volatile impurities by gas chromatographic method

Researchers have been experimenting with alternative fuels due to steadily increasing demand and decreasing supply of petroleum crude oil. Ethanol has been recognized as a possible liquid fuel alternative which can be available in significant quantities throughout the remainder of this century [1].
Ethanol blending is a process of mixing ethanol with petrol to enhance the octane content in fuel. This procedure reduces engine carbon monoxide (CO) and carbon dioxide emissions by up to 30 %. Moreover, it improves engine operation since it acts as an anti-knock agent [2].
Researchers have used ethanol as a component in conventional petroleum fuels because it has a higher thermal efficiency than ordinary petroleum motor fuels. Another benefit is its lower risk of fire during storage and transportation compared to petroleum, which has greater volatility and lower flash point than ethanol.
The EN 15721 European Standard relates to the determination of several compounds that may be present in ethanol at different concentration levels. The target compounds are separated into the following groups:
- Higher alcohols:
propan-1-ol, butan-1-ol, butan-2-ol, 2-methylpropan-1-ol (isobutanol), 2-methylbutan-1-ol, 3-methylbutan-1-ol. The compounds of the first group can be determined up to 3 % (m/m). - Impurities:
ethanol (acetic aldehyde), ethyl-ethanoate (ethyl acetate), 1,1-diethoxy ethane (acetal) are specified up to 2 % (m/m).
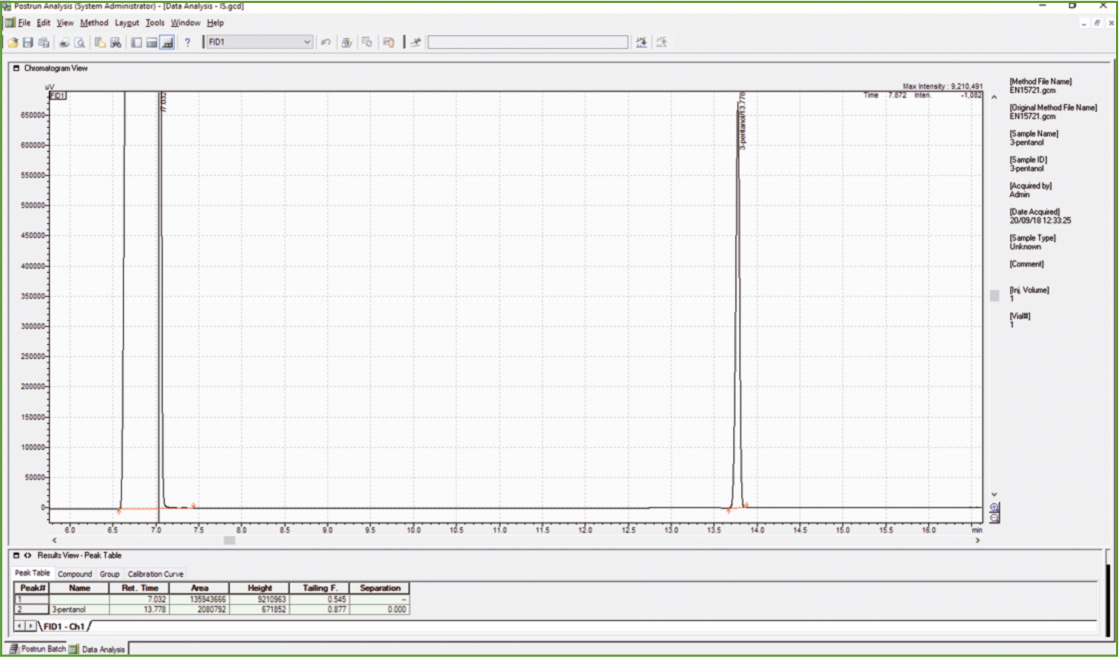 Figure 1: Chromatogram of 3-pentanol (ISTD)
Figure 1: Chromatogram of 3-pentanol (ISTD)
This gas chromatographic method includes the determination of a test portion with split injection mode into a gas chromatograph (GC) system. Addition of pentan-3-ol to the sample is the only sample pretreatment step. The sample is then introduced to the gas chromatograph (GC) system using split injection mode. A Flame Ionization Detector (FID) is used for the detection of the analytes.
 GC-2010 Plus
GC-2010 Plus
Apparatus
Gas chromatograph: Shimadzu GC 2010 Plus, Split/Splitless injector, FID detector
Capillary column: DB-1701 (60 m × 0.25 mm ID, 1.00 µm film thickness)
Two calibration stock solutions are used for the preparation of calibration standards and control samples. The first solution (catalogue number EN 15721-A) contains the ten target-compounds at 1 % (m/m) and the second (catalogue number EN 15721-A-IS) contains the internal standard (1 % m/m pentan-3-ol).
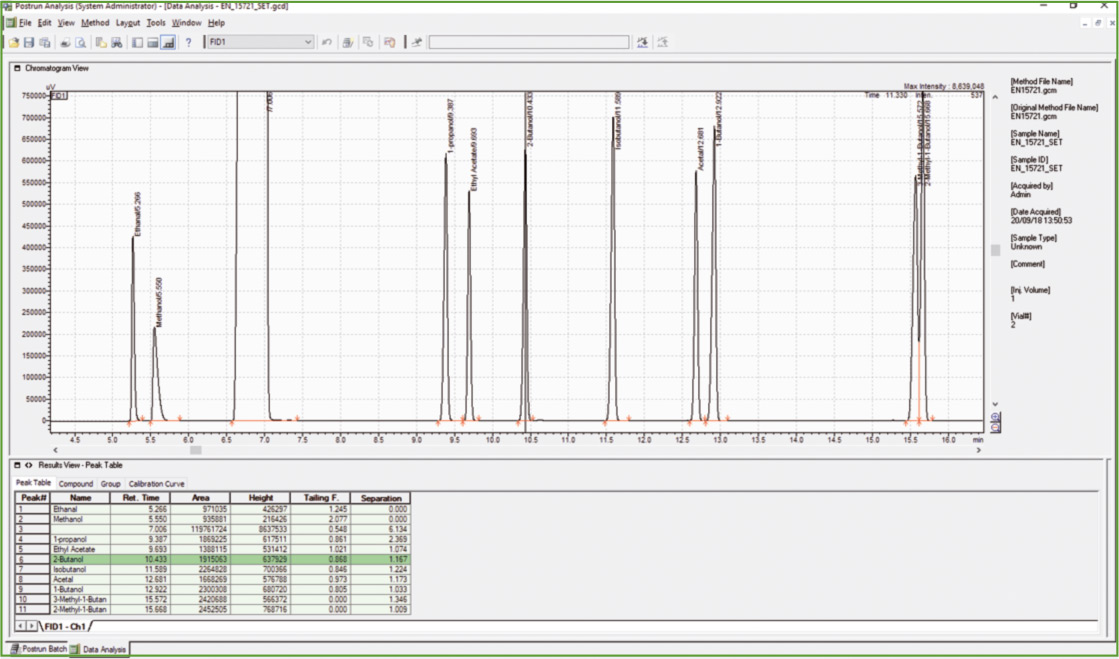 Figure 2: Chromatogram of calibration stock solution
Figure 2: Chromatogram of calibration stock solution
Calibration solution and sample preparation
Preparation of Calibration solution: 1 mL of ethanol is transferred into a 2 mL vial and is weighed to the nearest 0.1 mg. 100 uL of calibration stock solution (catalogue number EN 15721-A) is added and mass is recorded to the nearest 0.1 mg. 80 uL of internal standard stock solution (catalogue number EN 15721-A-IS) is added and mass is recorded to the nearest 0.1 mg [3].
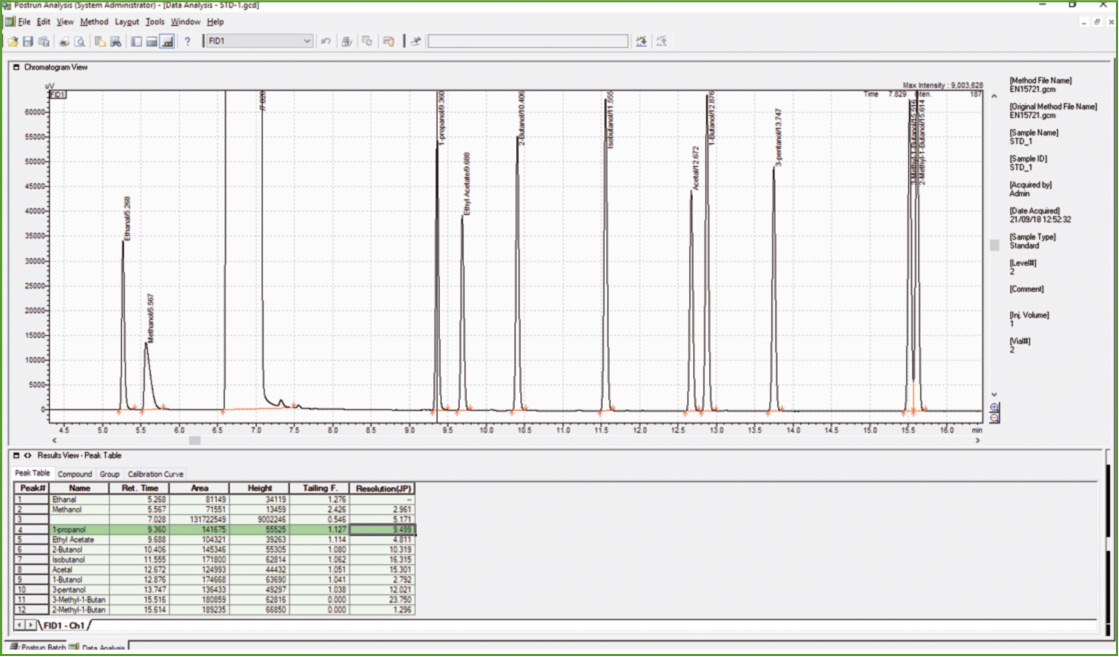 Figure 3: Chromatogram of calibration solution
Figure 3: Chromatogram of calibration solution
Sample Preparation: 1 mL of sample is transferred into a 2 mL vial and is weighed to the nearest 0.1 mg. 80 uL of internal standard stock solution (catalogue number EN 15721-A-IS) is added and mass is recorded to the nearest 0.1 mg [3].
Results and discussion
Chromatograms
The analysis was started with the injection of internal standard stock solution (catalogue number EN 15721-A-IS) and the injection of calibration stock solution since retention times of all compounds should be determined.
The next step was the analysis of Calibration Standard Solution to confirm compliance with the acceptance criteria, as far as chromatographic resolution is concerned. Baseline separation was obtained for all components except for 2-methylbutan-1-ol and 3-methyl-butan-1-ol (R = 1.3, R ≥ 1.0 is the EN 15721 specification).
Calibration curve
The Calibration Standard Solution was injected three times to obtain a two-point calibration curve (y = a x) for each analyte. The desired repeatability levels were achieved since the % RSD was 0.0 – 0.5 % (% RSD ≤ 5.0 is the EN 15721 specification) for both area ratio and response factor results of three calibration standard injections.
Expression of results
Results were calculated through the GC solution software according to the following equations:
Resolution: R = 2 (t2 – t1) / 1,699 (W1 + W2) Response Factor: RF = Area (ISTD) x C (compound) / Area (compound) x C (ISTD) Impurities: Q = ethanol + ethylethanoate + 1,1-diethoxyethane + oxygenated compounds Methanol: Cm = methanol content
Higher alcohols: Ch = propan-1-ol + butan-1-ol + butan-2-ol + 2-methylpropanol + 2-methylbutan-1ol +3-methylbutan-1-ol
Concentration is expressed in g/100 g, % (m/m).
Conclusion
The EN 15721 standard was successfully applied with the GC-2010 Plus. The appropriate use of the instrument and the accurate application of EN 15721 resulted in chromatograms with an acceptable number of theoretical plates while the presence of the AOC-20i auto-sampler contributed to the excellent repeatability.
Selection of an appropriate column was critical to achieve acceptable resolution (Rs > 1.0) between 2-methyl-1-butanol and 3-methyl-1-butanol. Also, the chromatographic method parameters of EN 15721 and the adoption of default values in gas flow rates for the detector (FID) ensured that all acceptance criteria of the Standard were fulfilled.
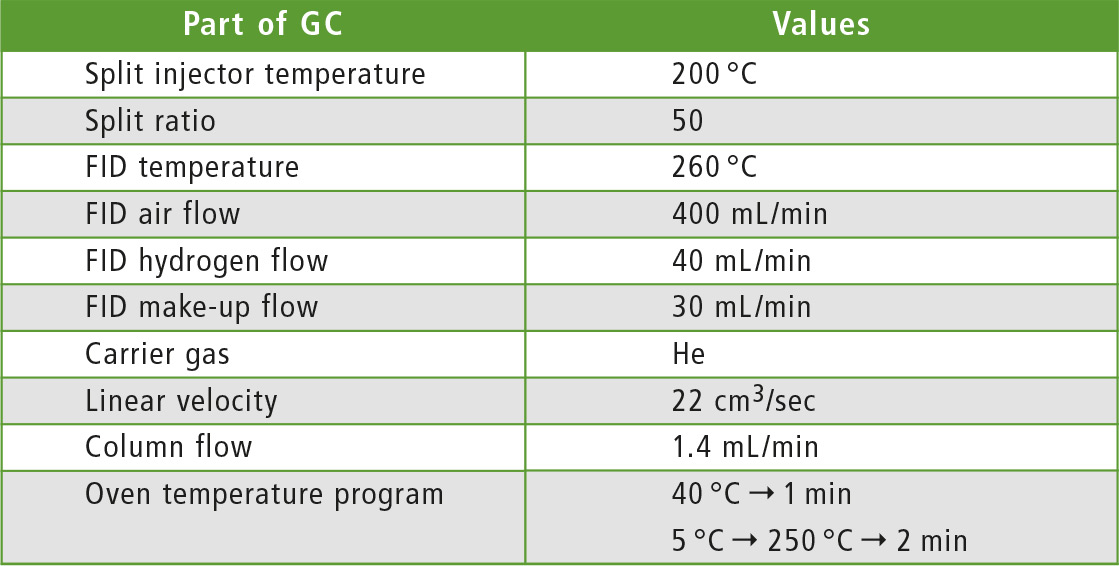 Table 1: Method parameters
Table 1: Method parameters
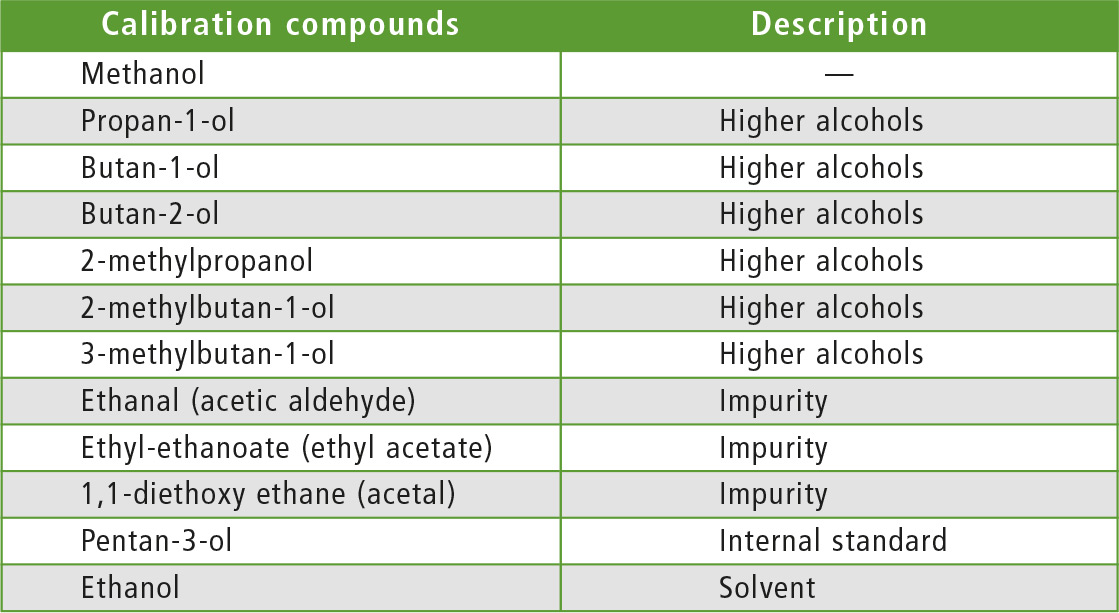 Table 2: Reagents and materials
Table 2: Reagents and materials
Finally, proper maintenance of the injection port and column oven before analysis helped to obtain chromatograms with low noise levels and high S/N values for the target-compounds.
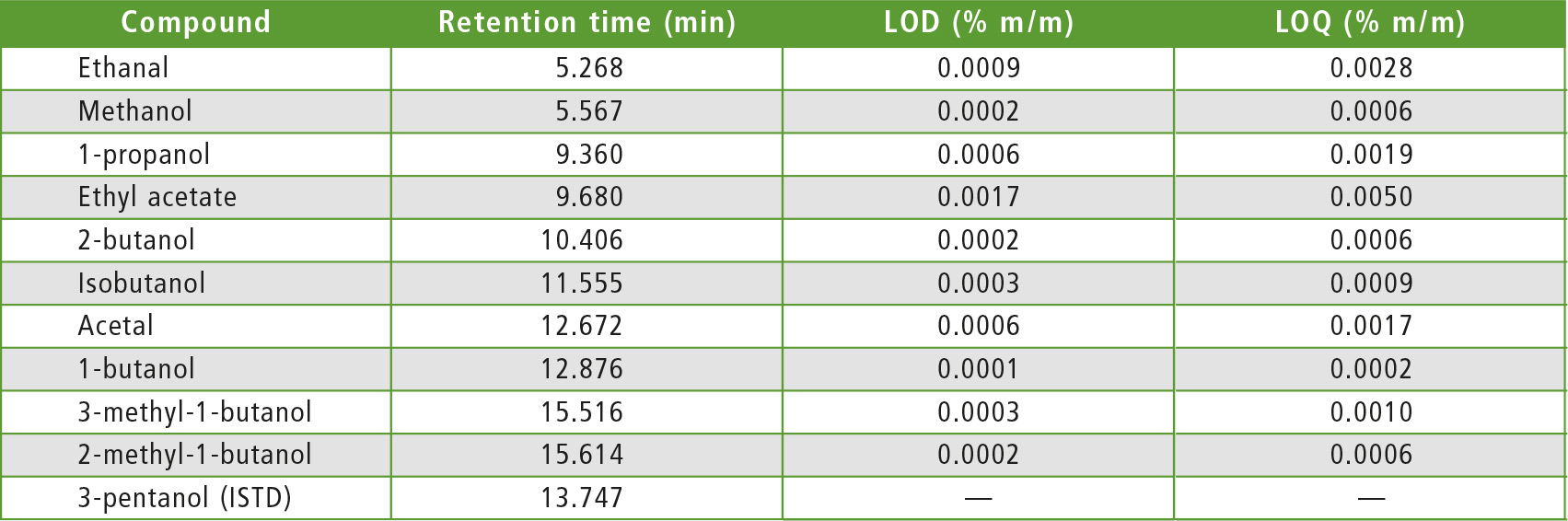 Table 3: Compound table
Table 3: Compound table

Authors
Fotis Fotiadis, Georgia Flessia, Dr. Gerasimos Liapatas, Dr. Manos Barbounis, N.Asteriadis S.A., Athens, Greece
Literature
[1] G. Najafi, B. Chobadian, T. Tavakoli, D.R. Buttsworth, T.F. Yusaf, M. Faizollhnejad, Appl. Energy 86 (2009) 630-639.
[2] Y. Barakat, Ezis N. Awad, V. Ibrahim, Egyptian Petroleum Research Institute (EPRI), Egypt (2016), “Fuel consumption of gasoline ethanol blends at different engine rotational speeds”
[3] EUROPEAN STANDARD EN 15721 “Ethanol as a blending component for petrol – Determination of higher alcohols, methanol and volatile impurities – Gas chromatographic method”