Which TOC technique is the best?
Cleaning Validation
 TOC-L with ASI-L autosampler
TOC-L with ASI-L autosampler
To ensure quality control and safety in manufacturing facilities within the pharmaceutical industry, it is important to apply cleaning validation. This technique is to be conducted after cleaning of production-related equipment in order to ensure that the quantity of residual substances collected from the equipments’ surfaces is within permissible limits. Three methods are available for cleaning validation using a TOC analyzer:
- Rinse sampling
- Swab sampling – aqueous extraction
- Swab sampling – direct combustion carbon measurement method
The methods are described here in detail using the TOC-LCPH total organic carbon analyzer (Shimadzu Corporation, Kyoto, Japan) for measurement of residual pharmaceutical products and their constituent substances.
Preparation of Residue Sample
To evaluate the cleaning validation sampling methods, residue measurement samples were created by applying various types of pharmaceutical products and their constituents to stainless steel pots. The aqueous and non-aqueous substances used are listed in table 1.
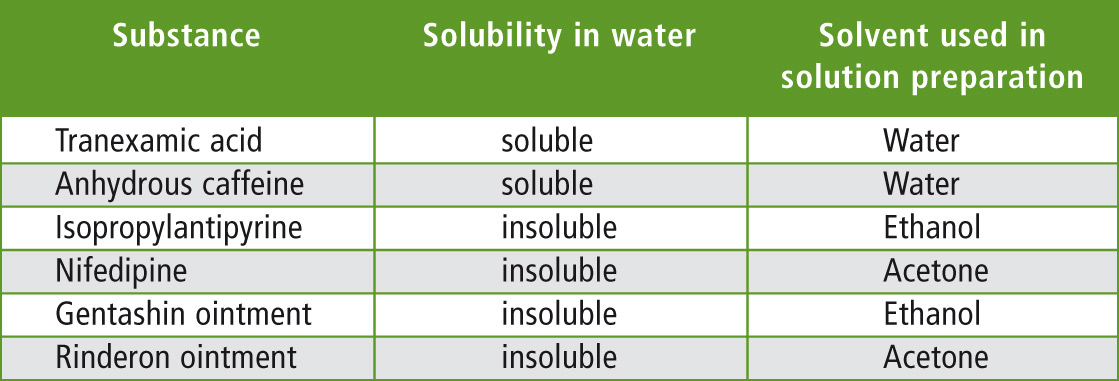 Table 1: Sample types
Table 1: Sample types
The aqueous and non-aqueous substances were dissolved in water and ethanol or acetone respectively, and the solution concentrations were adjusted to 2,000 mg C/L (= carbon concentration of 2,000 mg/L). Each residue substance measurement sample consisted of a 5 cm2 area on the surface of a stainless steel pot to which a volume of 100 µL of each solution was applied and dried. Carbon amount in the sample at each application site was therefore 200 µg. Among these, Gentashin ointment (aminoglycoside antibiotic) and Rinderon ointment (corticosteroid) were prepared based on determination of their carbon concentrations using the TOC analyzer equipped with solid sampling module.
1: Rinse Sampling
The Rinse Sampling method uses TOC measurement of the final rinse water used in the cleaning of a production equipment unit. This method is suitable for systems that cannot easily be disassembled, such as CIP (clean-in-place) equipment and narrow tubing. Sampling is considered to be difficult if the residues are not soluble in water.
To evaluate the recovery of the various substances when using the rinse sampling method, 100 mL of pure water was transferred to the stainless steel pot with its patch of dried sample, and after stirring for 15 minutes to prepare the rinse solution, TOC measurement was conducted.
Measurement Conditions
Analyzer: Shimadzu Total Organic Analyzer TOC-LCPH
Catalyst: High sensitivity catalyst
Measurement item: TOC (= TOC by acidification sparge processing)
Calibration curve: 2-point calibration curve using 0-3 mg C/L potassium hydrogen phthalate aqueous solution Injection volume: 500 µL
Since carbon content in each of the residue measurement samples was 200 µg, TOC concentration should be 2 mg C/L if all of the sample were to dissolve in the water.
For the blank, measurement was conducted in the same way using water transferred to a stainless steel pot with no dried sample applied to its surface. The blank concentration measured was subtracted from each TOC concentration and then compared with the theoretical value of 2 mg C/L to determine the rate of recovery. The results are shown in table 2.
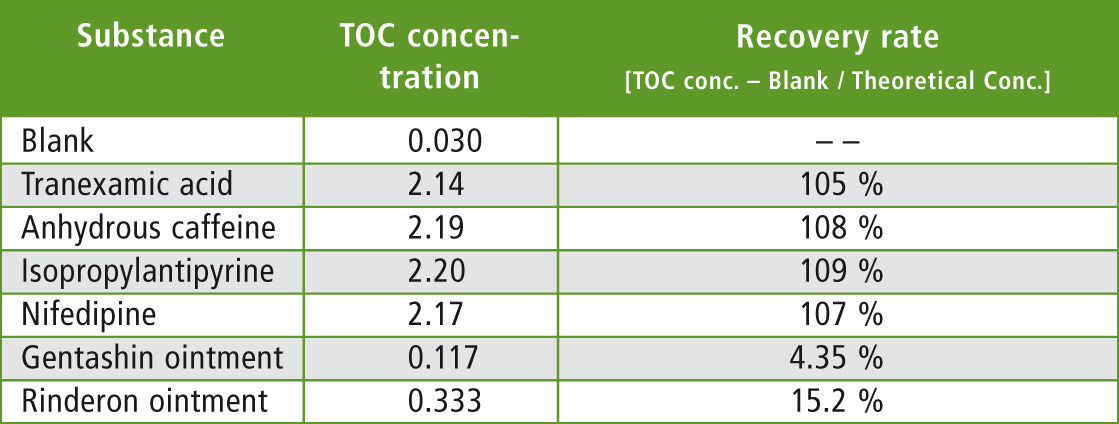 Table 2: Measurement results for Rinse Sampling – TOC Measurement Method
Table 2: Measurement results for Rinse Sampling – TOC Measurement Method
Water-soluble tranexamic acid and water-insoluble anhydrous caffeine had high recovery rates as expected. Water-insoluble isopropylantipyrine and nifedipine also had high recovery rates.
However, recovery rates of Gentashin ointment and Rinderon ointment were both low at less than 20 %. Based on these results, it is clear that evaluation of the rinse water using this method is unreliable due to the variation of recovery of substances which are not readily soluble in water.
2: Swab Sampling – Water Extraction
The Swab Sampling Water Extraction method consists of wiping the inside surface of the production apparatus with a fibrous swab material, extracting the adhering material with water, and conducting TOC measurement of the extract solution. Since the residue is physically wiped from a fixed area of the surface of the apparatus using the swab material and then analyzed, sampling efficiency is high. Since however water is used for extraction of the residue, residues that are insoluble in water are difficult to extract. Accordingly, cleaning evaluation with respect to these residues may be difficult for the same reasons as the Rinse Sampling method.
To evaluate recovery of the various substances when using the Swab Sampling Water Extraction method, the sample, which was applied to a stainless steel pot was wiped off with a 5 cm2 piece of fibrous swab material, which was then placed in a glass jar containing 100 mL of pure water. The residue was then extracted by stirring for one hour, after which TOC measurement was conducted. Since the fibrous swab material used (Alpha 10 obtained from Texwipe Co.) consists of polyester, very little organic material was extracted from the swab itself.
Measurement Conditions
Analyzer: Shimadzu Total Organic Analyzer TOC-LCPH
Catalyst: High sensitivity catalyst
Measurement item: TOC (= TOC by acidification sparge processing)
Calibration curve: 2-point calibration curve using 0-3 mg C/L potassium hydrogen phthalate aqueous solution
Injection volume: 500 µL
Swab material: 5 cm2 piece of Texwipe Alpha 10 swab material washed in pure water and dried
Since the carbon content in each of the residue measurement samples was 200 µg, TOC concentration in the extraction solution would be 2 mg C/L if all of the sample were wiped off.
For the blank, measurement was conducted in the same way by wiping the stainless pot, which had no sample applied before conducting extraction. The measured blank concentration was subtracted from each TOC concentration and then compared with the theoretical value of 2 mg C/L to determine the rate of recovery. Results are shown in table 3.
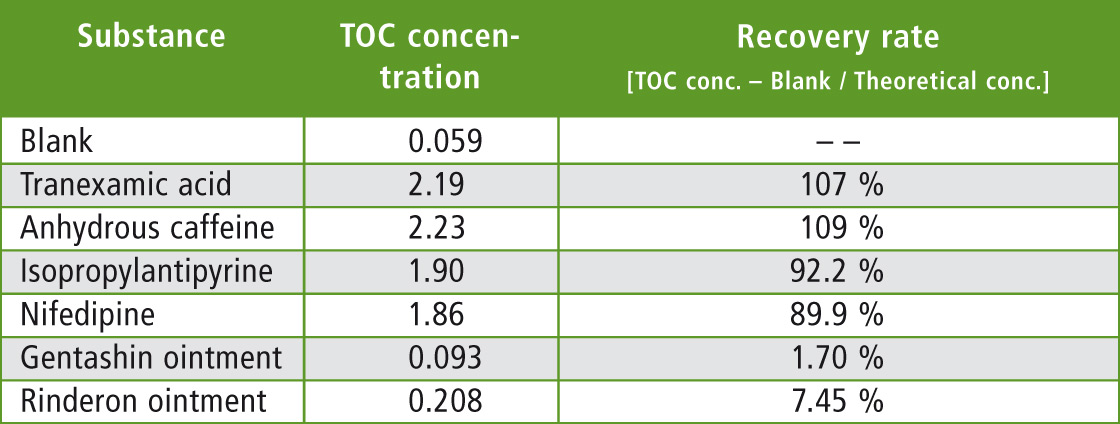 Table 3: Measurement results for Swab Sampling – Water Extraction TOC Measurement Method
Table 3: Measurement results for Swab Sampling – Water Extraction TOC Measurement Method
As expected, water-soluble tranexamic acid and water-insoluble anhydrous caffeine had high recovery rates while water-insoluble isopropylantipyrine and nifedipine had high recovery rates of about 90 %. However, recovery rates of Gentashin ointment and Rinderon ointment were both low at less than 10 %. Based on these results it is clear that evaluation of the rinse water using this method is unreliable due to the variation of recovery of substances which are not readily soluble in water.
3: Swab Sampling Direct Combustion Method
The Swab Sampling Direct Combustion method consists of wiping the inside surface of the production apparatus with a piece of inorganic quartz filter paper swab material, and then conducting measurement using a direct combustion carbon measurement system. The swab material with adhering residue is simply placed in the sample boat and the carbon content is measured directly by the TOC analyzer with a connected Solid Sample Combustion Unit (SSM-5000A, Shimadzu Corporation, Kyoto, Japan). Using this method, water-insoluble residues that are difficult to extract in water can also be collected, and measurement can be conducted quickly and easily without the need for any pretreatment such as sample extraction or similar.
To evaluate the rate of recovery of the different types of substances using the Swab Sampling – Direct Combustion method, quartz glass filter paper swab material was used to wipe the sample adhering to the stainless steel pot and placed in the SSM-5000A sample boat. TC measurement was then conducted.
Measurement Conditions
Analyzer: Shimadzu Total Organic Analyzer TOC-LCPH + SSM-5000A Solid Sample Combustion Unit (IC circuit bypass using system with cell switching valve set)
Cell length: Short cell
SSM carrier gas: 400 mL/min oxygen gas
Measurement item: TC Calibration curve: 1-point calibration curve using 1 % C glucose aqueous solution
Swab material: Advantec quartz glass paper QR-100 (diameter 45 mm) heat-treated at 600 °C for 15 minutes
Since carbon content in each of the residue measurement samples was 200 µg, the TC value would be 200 µg if all of the sample were wiped off. For the blank, measurement was conducted in the same way by wiping a stainless pot with no sample applied. The measured blank value was subtracted from each TC value and then compared with the theoretical value of 200 µg to determine the rate of recovery. Results are shown in table 4. A high recovery rate of about 100 % was obtained for all substances regardless of whether they were water-soluble or water-insoluble.
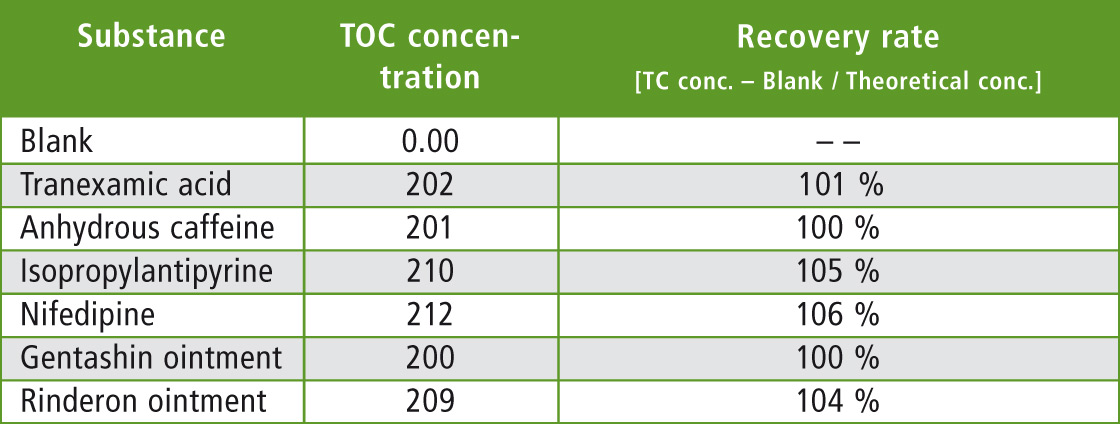 Table 4: Measurement results for Swab Sampling Direct Combustion Method
Table 4: Measurement results for Swab Sampling Direct Combustion Method
Conclusion
The methods used here and their respective recovery rates are summarized in table 5. When using the Rinse Sampling and the Swab Sampling Water Extraction methods, substances that do not dissolve easily in water were found to include those having both high and low recovery rates. It is thought that this may be due to differences in the strength with which the substances adhere to the stainless steel pot. Accordingly, it is probable that residue evaluation using these methods would be difficult for substances with low recovery rates.
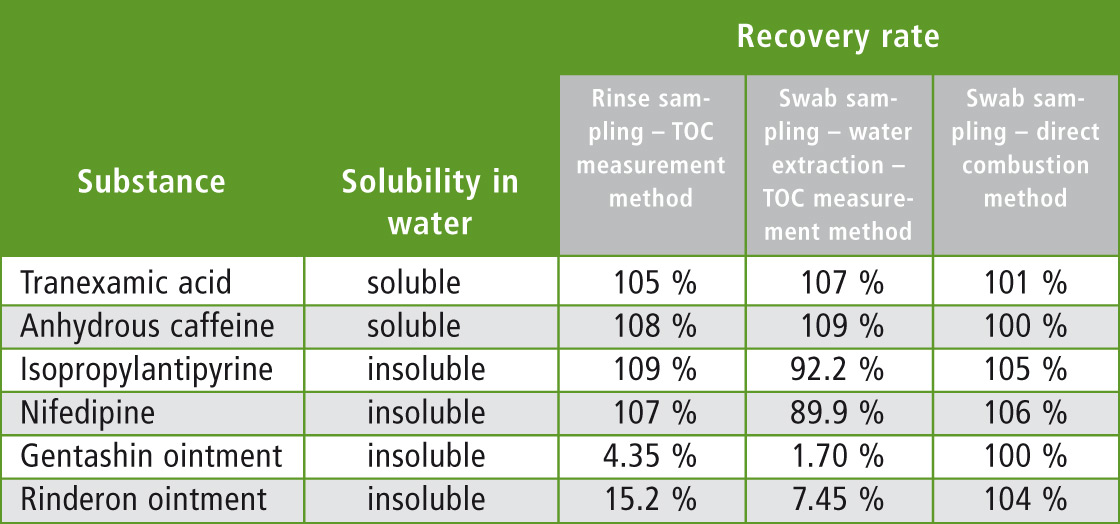 Table 5: Summary of measurement results
Table 5: Summary of measurement results
In contrast, high recovery rates were obtained for all substances when using the Swab Sampling Direct Combustion method, regardless of whether the substances were water-soluble or water-insoluble and thereby permitting residue evaluation. This method is therefore considered to be the most effective measurement method for conducting cleaning validation, especially when multiple compounds are being manufactured in the same vat.