MS detection of Alzheimer’s blood-based biomarkers
From a few drops of blood – the first successful detection of plasma Aβ by MS
Dr. Naoki Kaneko, Shimadzu Corporation
Amyloid β (Aβ) accumulation in the brain is considered as the earliest sign of Alzheimer’s disease (AD) pathology. Recently developed disease-modifying therapies (DMTs) target patients with early-stage AD, hence blood-based biomarkers are needed for a detection of Aβ accumulation. We combined immunoprecipitation (IP) with mass spectrometry (MS) to develop an IP-MS technique that resulted in the first successful detection of plasma Aβ by MS. This technique revealed a high concordance between a composite biomarker of two plasma Aβ ratios and amyloid PET status. As advances are made in DMTs, blood-based biomarkers will become increasingly valuable due to their key role for screening patients, monitoring drug effects and diagnosis.
Plasma amyloid β biomarker assay – creating the early bird for AD diagnosis
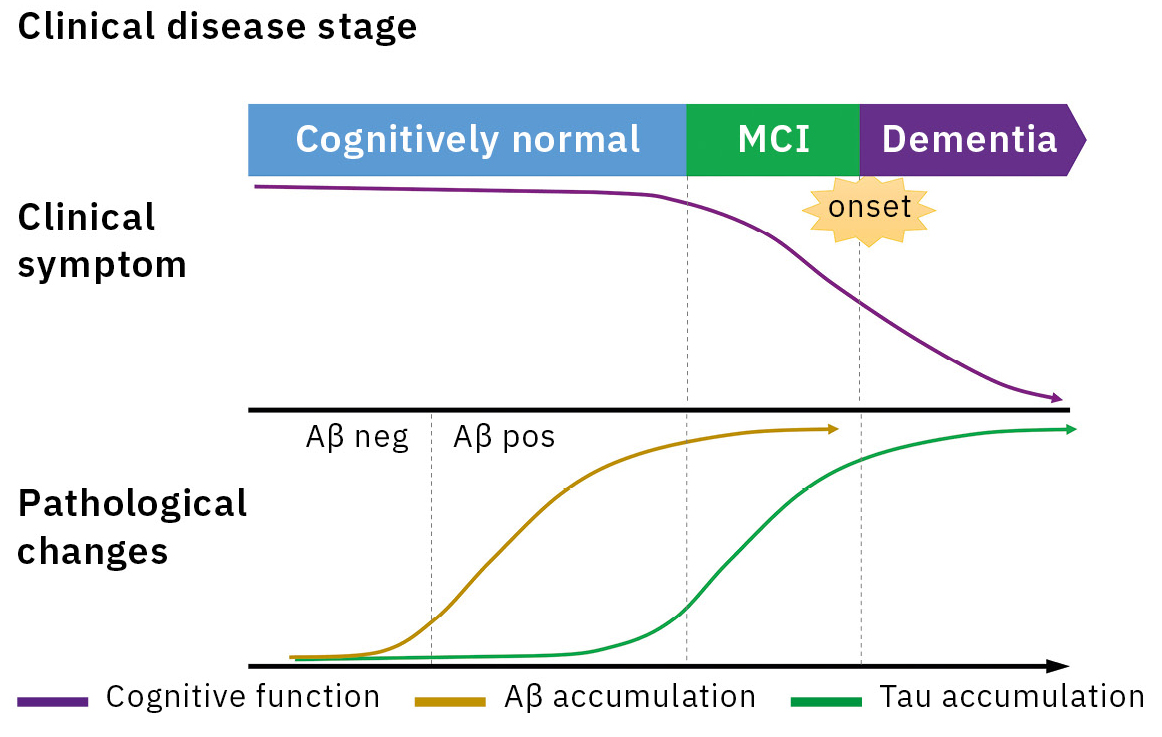
Alzheimer’s disease (AD) is the main cause of dementia, accounting for 60 to 70 % of all dementia cases. An accumulation of amyloid β (Aβ) in the brain is considered as the earliest sign in the continuum of AD pathological changes and starts 20 to 30 years prior to symptom onset (Figure 1).[1] Therefore, a biomarker reflecting the AD pathology plays a key role in the early diagnosis of AD. The detection methods for Aβ biomarker were amyloid positron emission tomography (PET) and immunoassay for cerebrospinal fluid (CSF) Aβ1-42 and Aβ1-42/1-40.
However, amyloid PET requires a large PET equipment and is expensive, and CSF Aβ causes more invasiveness of collecting CSF. Therefore, there was a need for a blood-based biomarker that can be measured easily. In recent years, the clinical utility of plasma biomarkers has been reported with the significant technological advancements for plasma biomarker measurement. Main technologies that measure plasma Aβ are an immunoprecipitation coupled with mass spectrometry (IP-MS) and a sandwich immunoassay employing two kinds of antibodies. A head-to-head study for comparison of plasma Aβ assays has reported a higher accuracy in IP-MS than in the immunoassay.[2, 3, 4]
In the following passage, amyloid MS technique using IP-MS methodology is highlighted with matrix-assisted laser desorption/ionization time of flight MS
(MALDI-TOF MS) and high performance of plasma Aβ biomarkers in clinical studies.
Amyloid MS: IP-MS methodology using MALDI-TOF MS
Immunoassays have been used in plasma Aβ biomarker research since 1996. However, many studies of plasma Aβ as a biomarker produced conflicting reports regarding the status of plasma Aβ due to its analytical difficulty, resulting in sceptical views about its utility for many years.[5] MS detects different peptides by separating them according to the mass with high sensitivity. Thus, MS can accurately and simultaneously detect even similar peptides such as Aβ1-38, Aβ1-40, Aβ1-42, etc. However, when a sample contains large amounts of impurities, the strong signal from these impurities drowns out the weak signal from analytes only present in small amounts. Blood plasma samples typically contain large amounts of impurities. This has prevented the use of mass spectrometry to measure biomarkers in plasma samples with high sensitivity.
The introduction of an antibody-based immunoprecipitation (IP) step into the sample preparation enabled a great success: selectively separating and concentrating only Aβ. An IP-MS method for detecting Aβ was already reported in 1996 and could readily detect Aβ in samples of cell culture supernatant and CSF,[6] but blood has many more impurities and lower Aβ concentrations than culture supernatant and CSF, preventing the detection of Aβ in blood by IP-MS.
Refining the conditions for IP enabled the first detection of Aβ1-42 and Aβ1-40 in human plasma
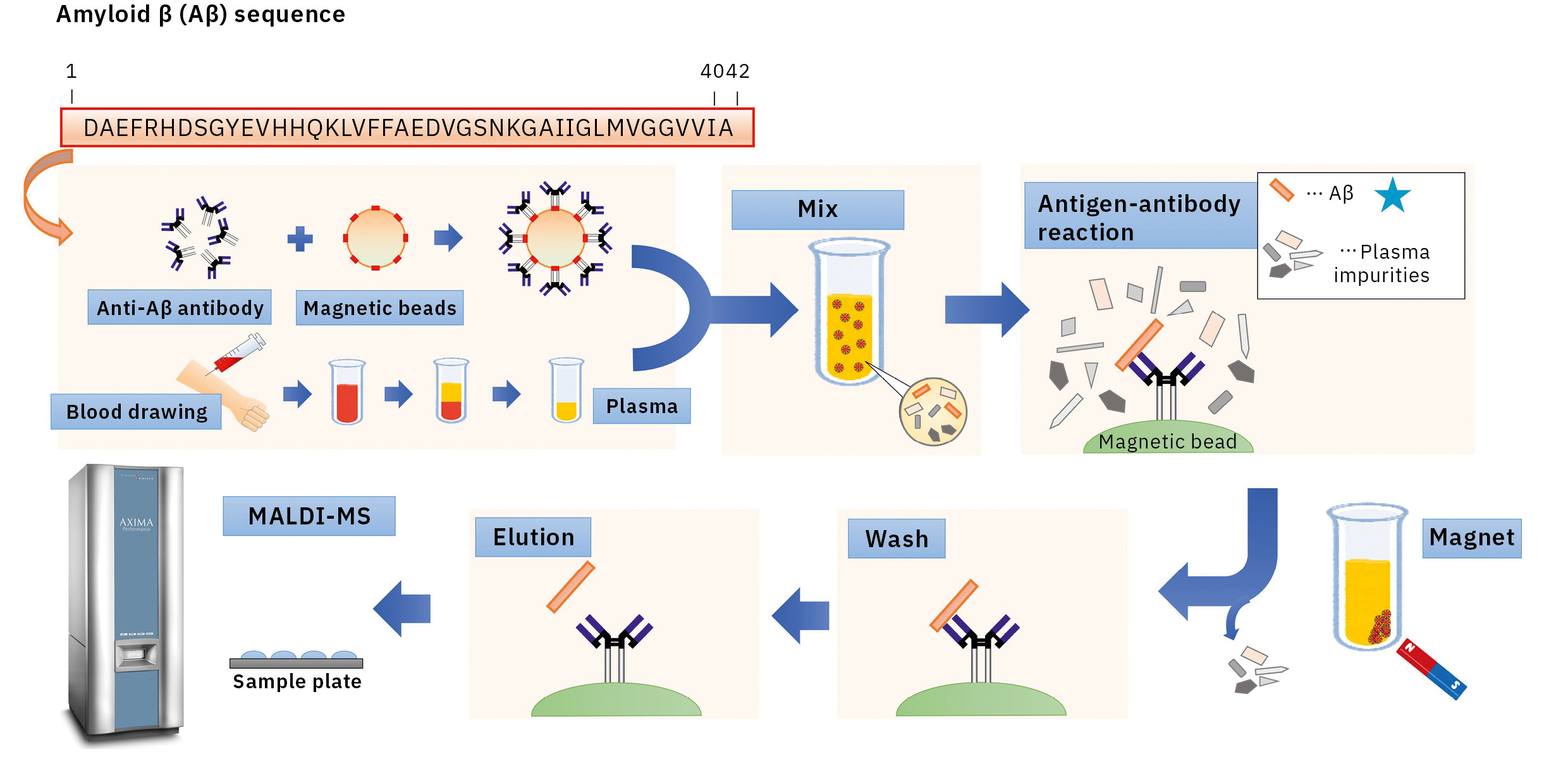
Optimized conditions used during IP helped to overcome these issues. After examining the preparation of antibody-coated beads, surfactants and eluate compositions during IP, a matrix solution composition was selected, appropriate for samples after IP to establish IP-MS methodology for plasma Aβ analysis using MALDI-TOF MS (AXIMA Performance) (Figure 2).
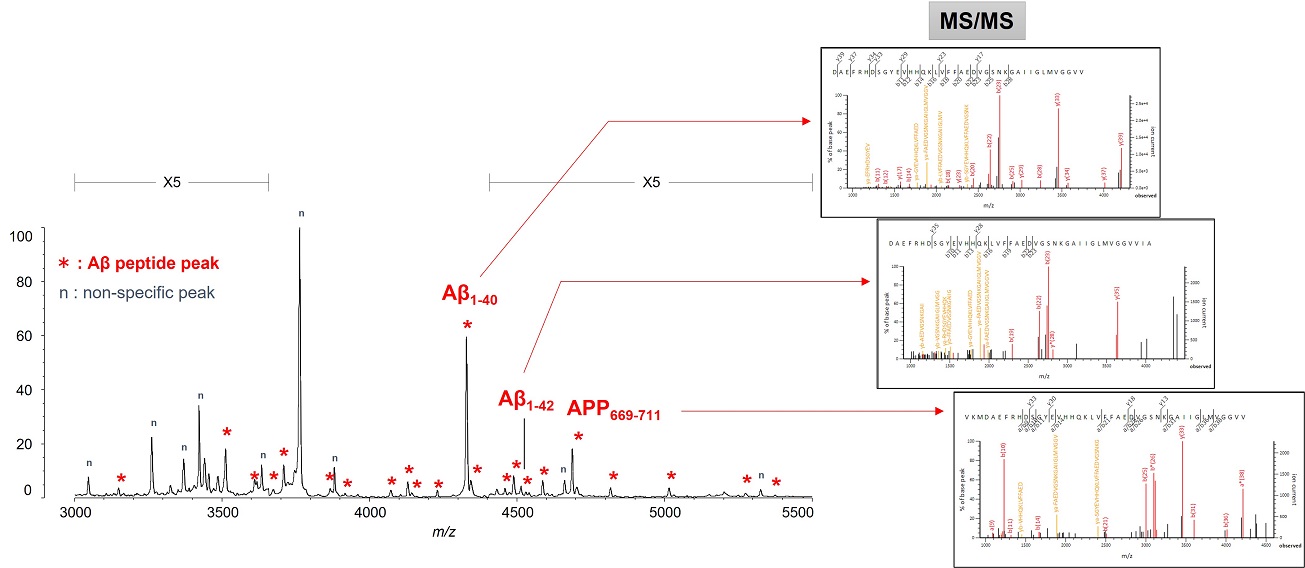
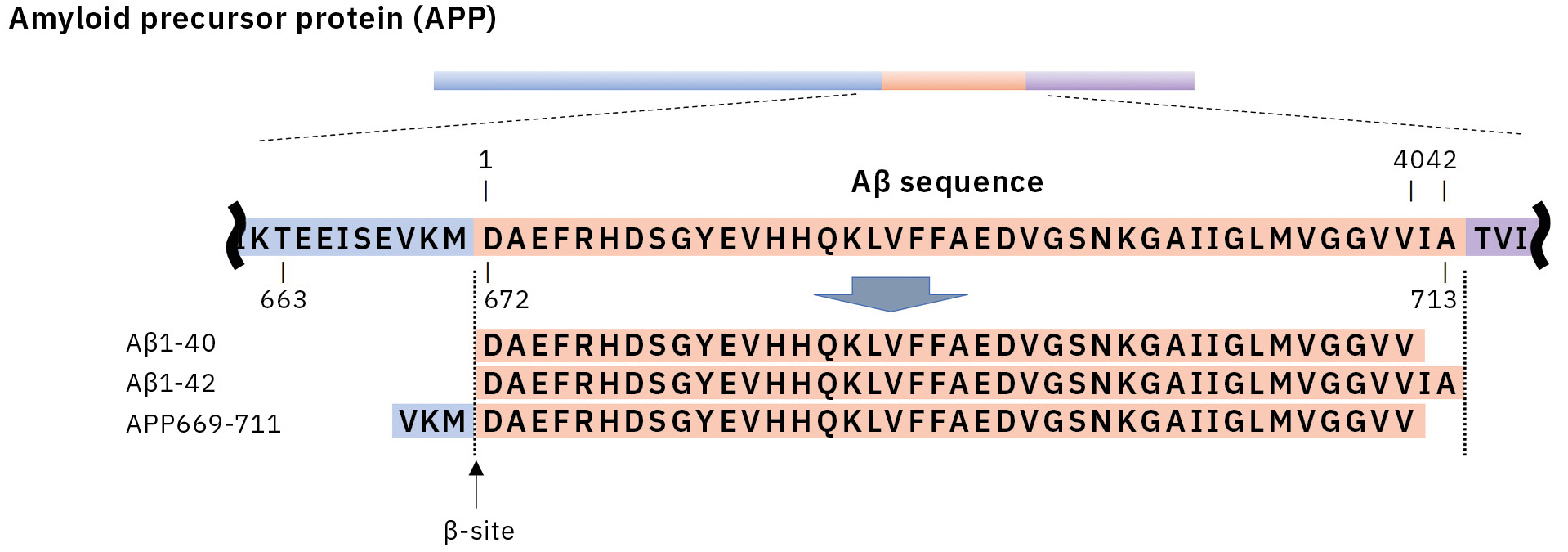
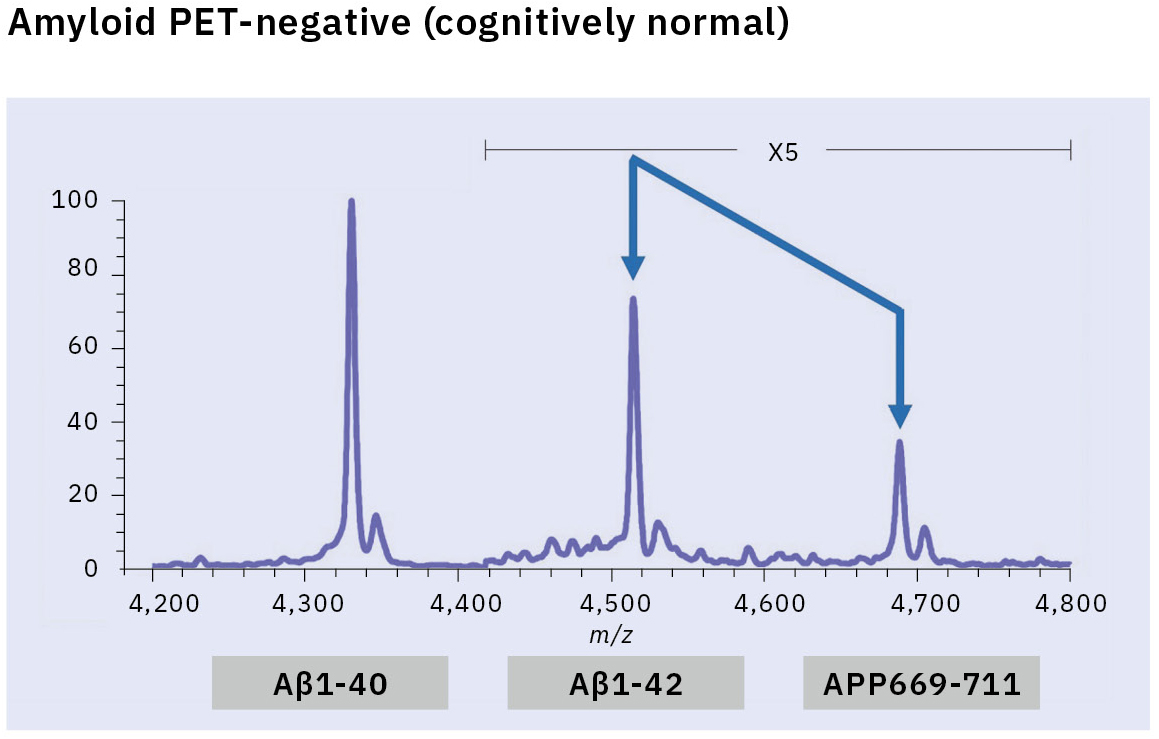
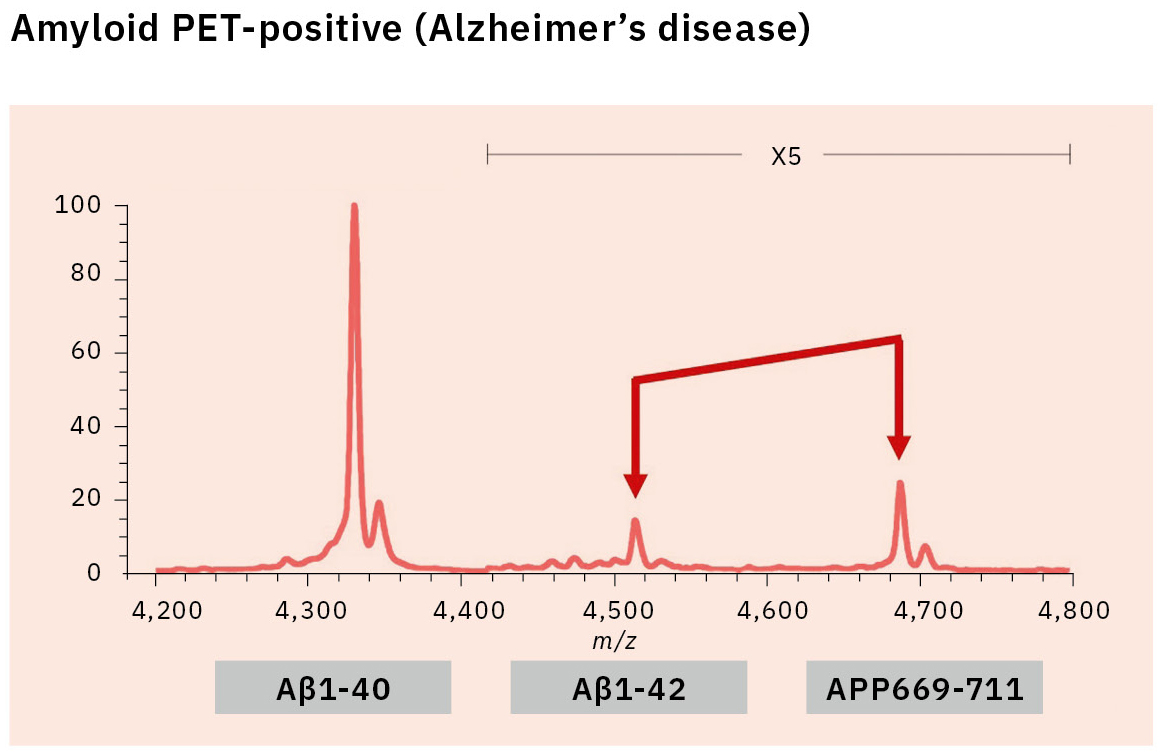
This resulted in the first successful detection of endogenous Aβ1-42 and Aβ1-40 in human plasma by MS and simultaneously produced other discoveries only available using MS (Figure 3).[7, 8] Specifically, MS revealed the presence of many Aβ species other than Aβ1-42 and Aβ1-40 in human plasma, including APP669-711 with an N-terminus elongated beyond Aβ1-x (Figure 4). In this IP-MS procedure using MALDI-TOF MS, the Aβ species after IP is directly applied to MALDI-TOF MS without protease digestion. This makes it possible to save the sample preparation time and measure intact Aβ species present in plasma.
Extensive blood-based biomarker studies
The research for an Aβ biomarker by IP-MS commenced in 2013 in cooperation with Japan’s National Center for Geriatrics and Gerontology. This biomarker discovery study using IP-MS and amyloid PET imaging with PIB (62 cases) detected a higher ratio of APP669-711 to Aβ1-42 (APP669-711/Aβ1-42) in plasma from amyloid PET-positive patients (Figure 5).[9] Receiver operating characteristic (ROC) analysis of the APP669-711/Aβ1-42 ratio for amyloid PET-positive patients also showed a high area under the curve (AUC) value of 96.9 %. The APP669-711/Aβ1-42 ratio was also significantly correlated with the standardized uptake value ratio (SUVR) of amyloid PET, with a correlation coefficient of 0.687 (p < 0.001). Based on this data, a high concordance between plasma APP669-711/Aβ1-42 ratio and amyloid PET was reported for the first time in 2014.
Insufficient reproducibility is a common issue with newly discovered blood-based biomarkers that often leads to their abandonment. Therefore, the blood-based biomarker required a validation using specimens that were entirely independent of those used in the discovery study. A validation study was conducted in two datasets from Japan’s National Center for Geriatrics and Gerontology and the Australian Imaging, Biomarker & Lifestyle Flagship Study of Aging (AIBL). ROC analysis of the APP669-711/Aβ1-42 ratio for amyloid PET-positive cases (using the PIB tracer) revealed a high AUC value of 92.3 % in the Japanese dataset (121 cases) and 89.5 % in the Australian dataset (111 cases) (Table 1).[10] A composite biomarker created by combining APP669-711/Aβ1-42 ratio and Aβ1-40/Aβ1-42 ratio (a well-known biomarker) also improved AUC values to 96.7 % in the Japanese dataset and 94.1 % in the Australian dataset. The concentration of Aβ1-42 in CSF is known to decrease when Aβ accumulates in the brain, a phenomenon that was similarly identified in the data, with reduced plasma levels of Aβ1-42 in amyloid PET-positive cases. This change of Aβ1-42 may be caused by Aβ1-42 being trapped in the brain, which lowers the amount of Aβ1-42 available to enter bodily fluids.
ROC analysis of the blood concentration of Aβ1-42 alone also showed AUC values of 87.2 % (Japanese dataset) and 75.7 % (Australian dataset), indicating Aβ1-42 alone was inadequate as a biomarker, though these AUC values increased when Aβ1-42 concentration was taken as a ratio of other Aβ species. This increase in AUC is probably due to individual variations in the total concentration of all Aβ species in the blood, which lowers the effectiveness of using absolute Aβ1-42 concentration alone as a biomarker. Taking Aβ1-42 as a ratio of another Aβ species may suppress the influence of this individual variation in total Aβ. This report published in 2018 showed a high concordance between plasma Aβ measured by IP-MS and amyloid PET and served as a trigger for the recognition of blood Aβ as a useful biomarker by researchers worldwide.
Less invasive biomarkers reflecting AD pathology lead the way to AD drug discovery
Amyloid MS technique can measure unique APP669-711/Aβ1-42 ratio as well as Aβ1-40/Aβ1-42 ratio in plasma. The Aβ composite biomarker created by combining the two Aβ ratios demonstrated high accuracy in distinguishing amyloid PET-positive cases from amyloid PET-negative cases. Biomarkers that reflect AD pathology are essential to AD drug discovery, diagnosis and staging, and blood-based biomarkers that provide a less invasive means of measuring large numbers of specimens will become increasingly useful.
|
Biomarker |
Evaluation |
NCGG (n=121) |
AIBL (n=111) |
|
Aβ1-42 |
AUC |
87.2% |
75.7% |
|
Sensitivity |
74.0% |
78.3% |
|
|
Specificity |
88.7% |
66.7% |
|
|
Accuracy |
82.6% |
73.0% |
|
|
APP669-711/Aβ1-42 |
AUC |
92.3% |
89.5% |
|
Sensitivity |
68.0% |
86.7% |
|
|
Specificity |
91.5% |
74.5% |
|
|
Accuracy |
81.8% |
81.8% |
|
|
Aβ1-40/Aβ1-42 |
AUC |
96.7% |
88.9% |
|
Sensitivity |
96.0% |
90.0% |
|
|
Specificity |
87.3% |
70.6% |
|
|
Accuracy |
90.9% |
81.1% |
|
|
APP669-711/Aβ1-42 |
AUC |
96.7% |
94.1% |
|
Sensitivity |
86.0% |
91.7% |
|
|
Specificity |
88.7% |
82.4% |
|
|
Accuracy |
87.6% |
87.4% |
[1] Villemagne V.L. et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12 (4): 357–367.
[2] Janelidze S. et al. (2021). Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol. 78 (11): 1375–1382.
[3] Brand A.L. et al. (2022). The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: a literature review. Alzheimers Res Ther. 14 (1): 195.
[4] Winston C.N. et al. (2023). Evaluation of Blood-Based Plasma Biomarkers as Potential Markers of Amyloid Burden in Preclinical Alzheimer’s Disease. J Alzheimers Dis. 92 (1): 95–107. doi: 10.3233/JAD-221118. PMID: 36710683.
[5] Shanthi K.B. et al. (2015). A systematic review and meta-analysis of plasma amyloid 1-42 and tau as biomarkers for Alzheimer’s disease. SAGE Open Med. 3: 2050312115598250.
[6] Wang R. et al. (1996). The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem. 271 (50): 31894–31902.
[7] Kaneko N. et al. (2014). Identification and quantification of amyloid beta-related peptides in human plasma using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Jpn Acad Ser B Phys Biol Sci. 90 (3): 104–117.
[8] Korecka M., Shaw L.M. (2021). Mass spectrometry-based methods for robust measurement of Alzheimer’s disease biomarkers in biological fluids. 159 (2): 211–233.
[9] Kaneko N. et al. (2014). Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B Phys Biol Sci. 90 (9): 353–364.
[10] Nakamura A. et al. (2018). High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 554 (7691): 249–254.