Improving biopharmaceutical drug development
Metal-free, high-quality LC-MS analysis of oligonucleotide therapeutics
Dr. Gesa Schad, Dr. Björn T. Erxleben, Shimadzu Europa GmbH
In recent years, oligonucleotide-based medicines have been recognized for their potential as new treatments for rare and chronic diseases and several new drugs have been approved by regulatory agencies. Analysis of these nucleic acid polymers to confirm identity and purity by LC-MS is a challenge, as they possess several reactive groups that can lead to adsorption onto metal surfaces in a stainless-steel-based UHPLC instrument. This article introduces the improvement of quantitative performance in the LC-MS analysis of oligonucleotides using the Nexera XS inert, a bioinert UHPLC system.
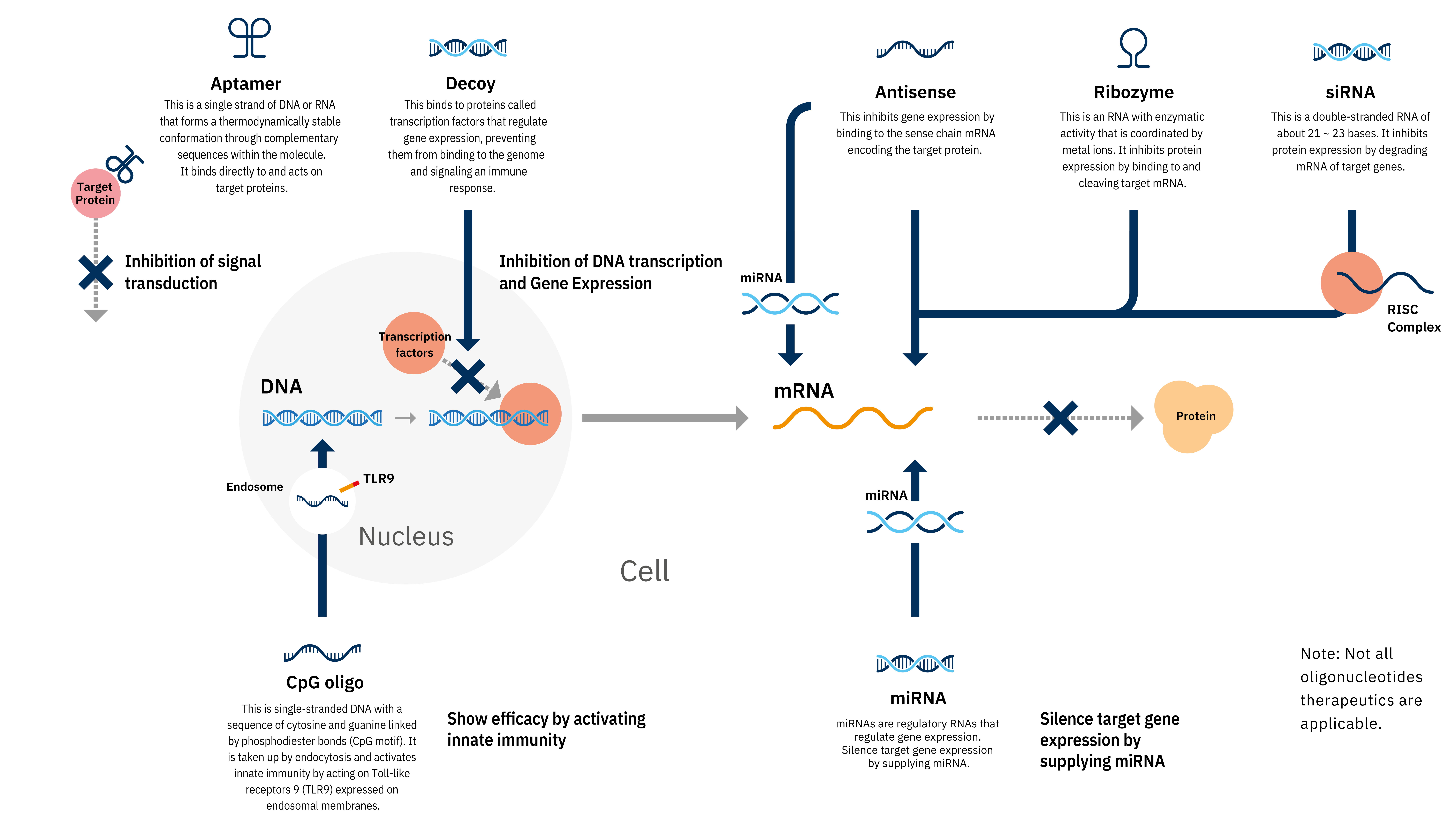
Oligonucleotide therapeutics are nucleic acid polymers generally comprised of a few to several dozen bases linked together. They are produced by chemical synthesis and act directly on organisms without being translated into proteins. Oligonucleotide therapeutics are characterized by the ability to target specific diseases and are part of the new wave of biopharmaceuticals – most visibly used in the quick and successful targeting of Covid-19. As depicted in Figure 1, the DNA oligomers bind to a target protein or RNA and have successfully been used to inhibit DNA transcription and gene expression. They have also shown efficacy by activating innate immunity. Another distinct advantage is that it takes less time than conventional methods to find new therapeutic candidates as oligonucleotides are straightforward to design and synthesize.
However, it is a practical problem that oligonucleotide therapeutics are degraded and excreted rapidly after administration by exonucleases and endonucleases that are abundant in blood and cells. This problem is being addressed by the introduction of modified oligonucleotides to improve chemical stability in vivo and by the development of lesion-targeted DDS (drug delivery system) technologies. The workflow for research and development of oligonucleotide therapeutics comprises target selection, oligomer synthesis, purification, characterization and quality control [1]. Thorough characterization and quality control by confirmation of molecular weight, concentration and the sequence of oligonucleotides is important to ensure safety and efficacy of a drug product, as it affects the recognition of target molecules.
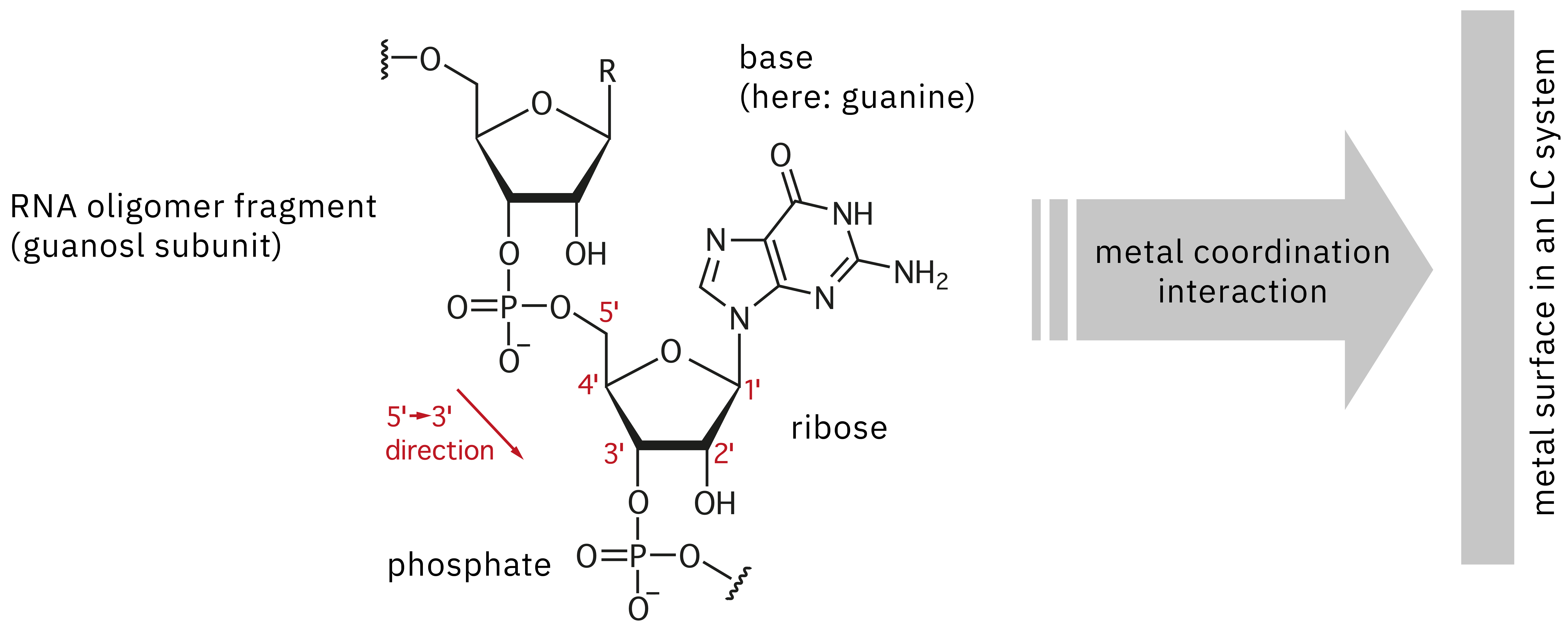
For evaluation of a synthesized drug product such as oligonucleotide therapeutics to confirm identity and absence of unwanted impurities, LC-MS precision mass spectrometry is the method of choice. However, analysis of these nucleic acid polymers poses some critical challenges, as they possess several reactive groups that can lead to adsorption onto metal surfaces in a stainless-steel-based UHPLC instrument (Figure 2). This interaction leads to poor peak shape, reduced sensitivity and lack of reproducibility. Repeat injection of a highly concentrated sample can be used to passivate a system before actually starting data acquisition, to “saturate” the adsorption sites on the surface and reduce the effect of sample loss. However, this approach not only wastes time and precious sample, it can also make it extremely difficult to acquire reliable data, due to changes in the state of passivation during continuous analysis. Alternatively, chelating agents could be used to suppress adsorption, but these are not applicable to LC-MS analysis, as they may lead to contamination of the ion source and loss in sensitivity. This article introduces the improvement of quantitative performance in the LC-MS analysis of oligonucleotides using the Nexera XS inert, a bioinert UHPLC system.
Experimental
The sample used in this analysis was an oligonucleotide with the sequence 5’-dG-dC*-dC*-dT-dC*-dA-dG-dT-dC*-dT-dG-dC*-dT-dT-dC*-dG-dC*-dC*-3’, where (*) indicates 5-C or 5-U methylation and (d) means 2’-deoxy nucleoside. The molecular weight was 6,431.72.
To evaluate the effect of using a bioinert system with no metal parts in the sample flow path, the analysis was performed using both a stainless-steel (SUS)-based UHPLC system (Nexera XR) with an SUS body separation column and the bioinert Shimadzu Nexera XS inert system using a metal-free column. Detailed analytical conditions are shown in Table 1. HFIP (1, 1, 1, 3, 3, 3-hexafluoro-2 propanol) and DIPEA (N, N-diisopropylethylamine) were used as ion-pair reagents, as is common practice in the reversed-phase analysis of oligonucleotides.
|
HPLC conditions (Nexera XR, Nexera XS inert) |
|
|
Column: |
Shim-pack Scepter C18-120 (100 x 2.1 mm, 3 µm) Shim-pack Scepter C18-120 (100 x 2.1 mm, 3 µm), metal-free |
|
Mobile phase A: |
50 mmol/L HFIP + 10 mmol/L DIPEA in water |
|
Mobile phase B: |
acetonitrile |
|
Flow rate: |
0.3 ml/min |
|
Gradient: |
5 %B (0—1 min), 5-30 %B (1—6 min), 95 %B (6.1—7 min), 5 %B (7.1—12 min) |
|
MS conditions (LCMS-8060) |
|
|
Ionization: |
ESI (negative mode) |
|
Probe voltage: |
-4 kV |
|
Mode: |
MRM (m/z 803.5 > 95.0) |
|
CID gas: |
330 kPa |
|
Nebulizing gas flow: |
3.0 L/min |
|
Drying gas flow: |
8.0 L/min |
|
Nebulizing gas flow: |
3.0 L/min |
|
Drying gas flow: |
8.0 L/min |
|
Heating gas flow: |
12.0 L/min |
|
DL temperature: |
300 °C |
|
Heat block temp.: |
450 °C |
|
Interface temp.: |
250 °C |
Results and discussion
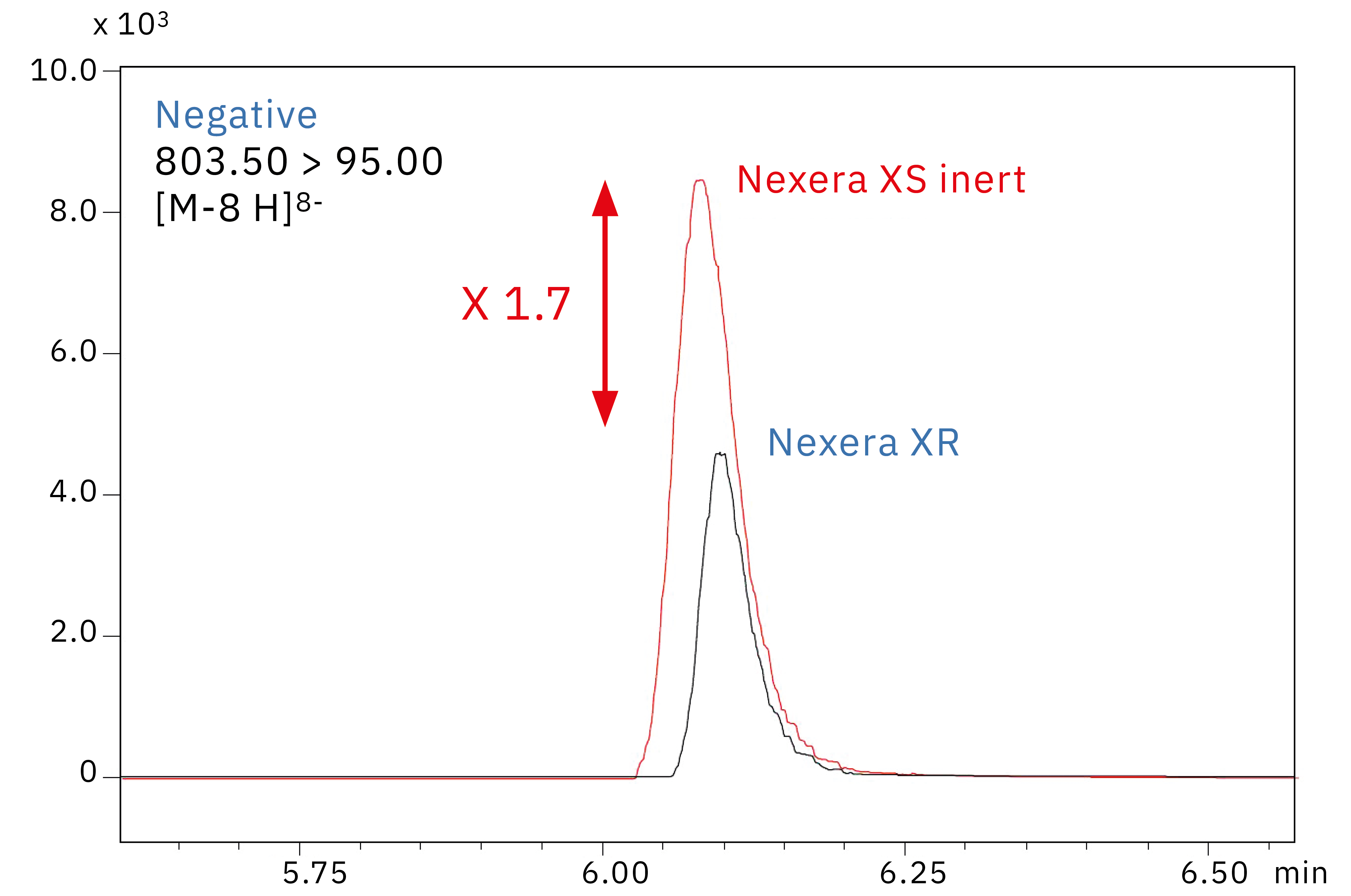
Figure 3 shows chromatograms obtained from the analysis of a 10 ng/mL oligonucleotide standard solution on a standard Nexera XR UHPLC system with an SUS-body column and on Shimadzu’s new Nexera XS inert system with a metal-free column. As can be clearly seen, there is a roughly 1.7-fold increase in sensitivity when metal is removed from the sample flow path.
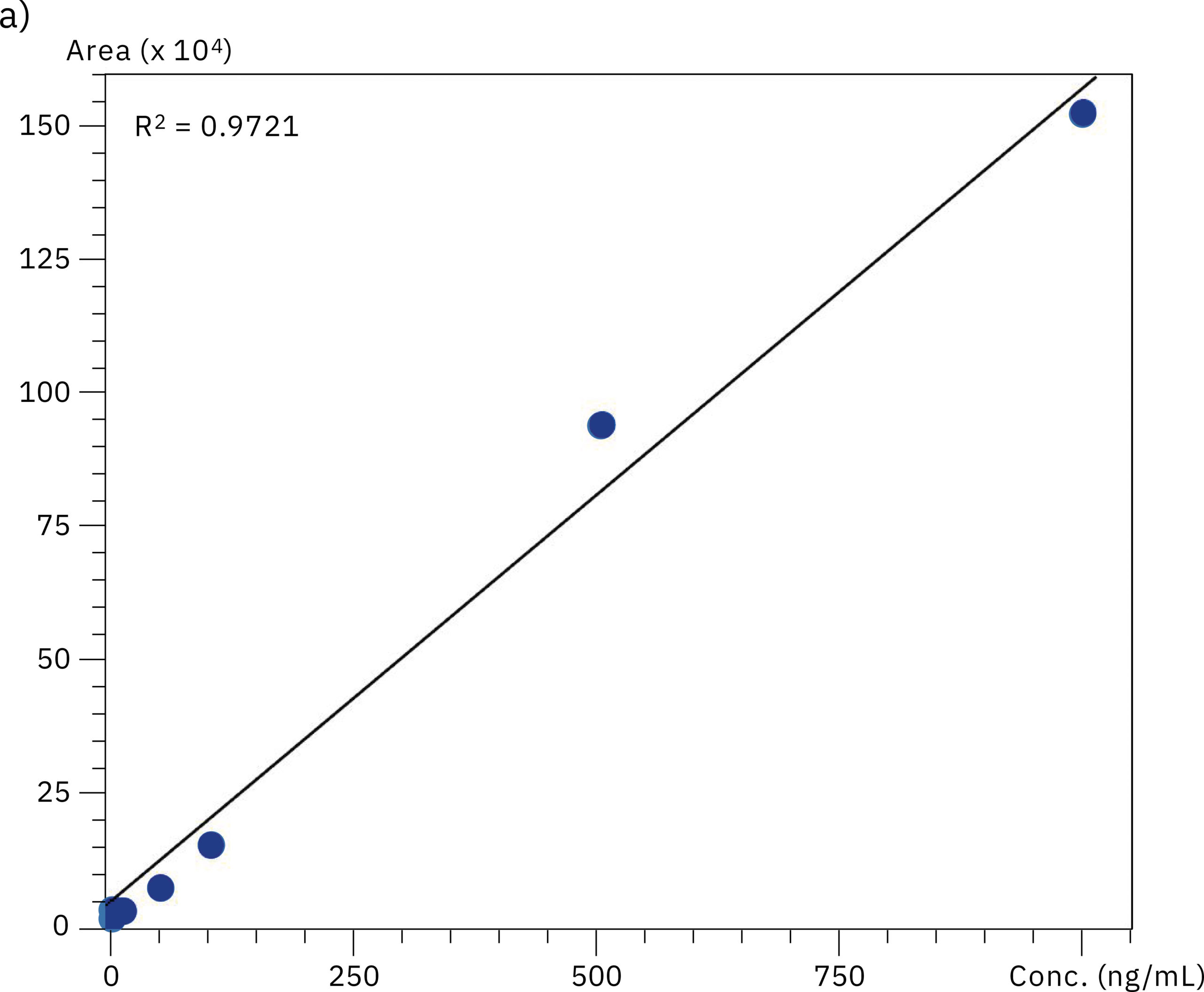
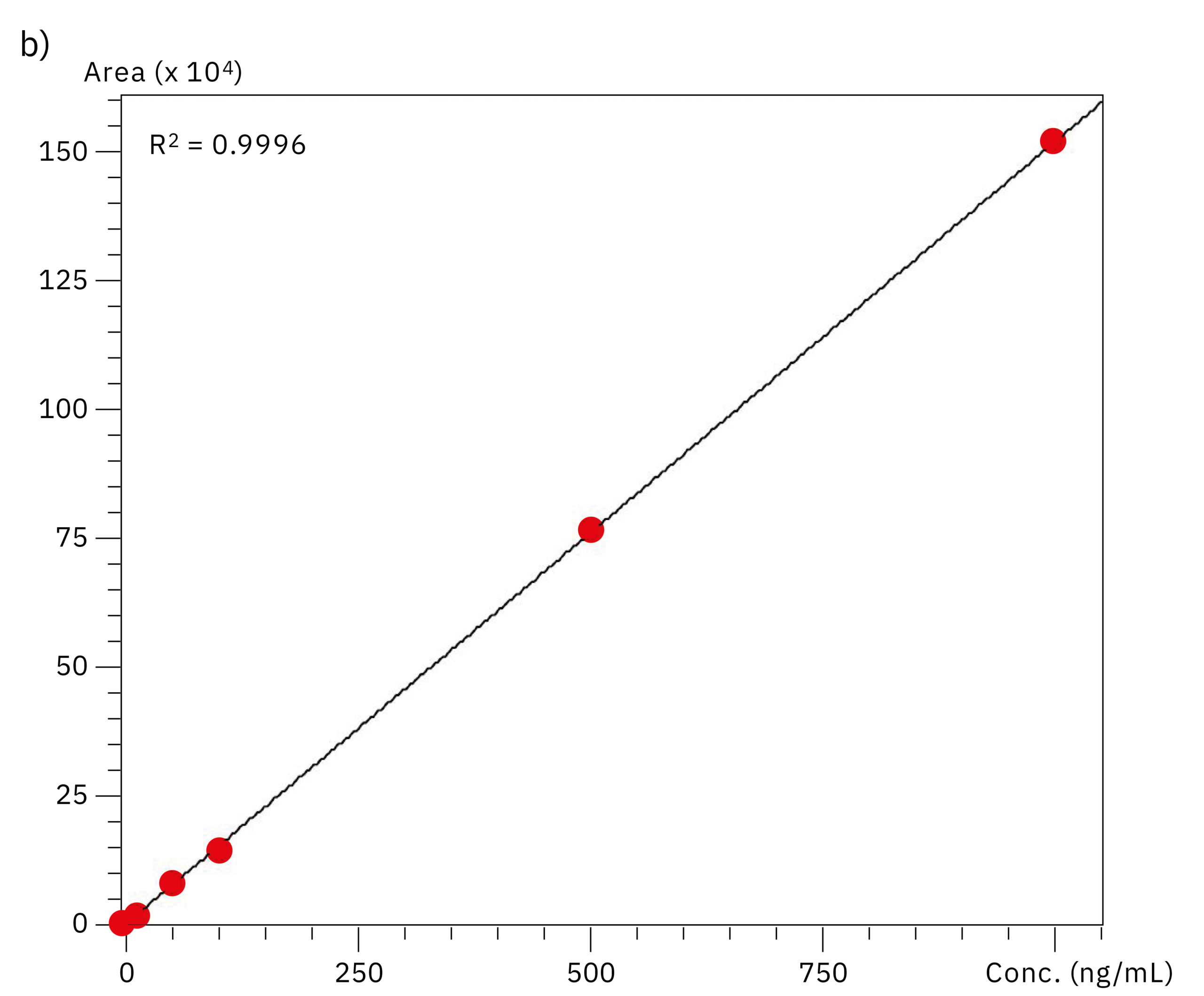
To compare the quantitative performance of the two systems for the analysis of oligonucleotides, calibration curves in the range of 0.5–1,000 ng/mL were produced. The graph obtained with data from the SUS-based Nexera XR system showed a lower value for linear regression (r2=0.9721) compared to the excellent linearity on the bioinert system (r2=0.9996), emphasizing the effect of metal adsorption on accuracy of the quantification in the highly sensitive LC-MS analysis of oligonucleotides. Results of the evaluation of linearity and accuracy at each calibration point can be found in Table 2 and Figure 4. These values highlight the improvement in reliability achieved when using the Nexera XS inert system with a metal-free separation column, especially when looking at very low sample concentrations.
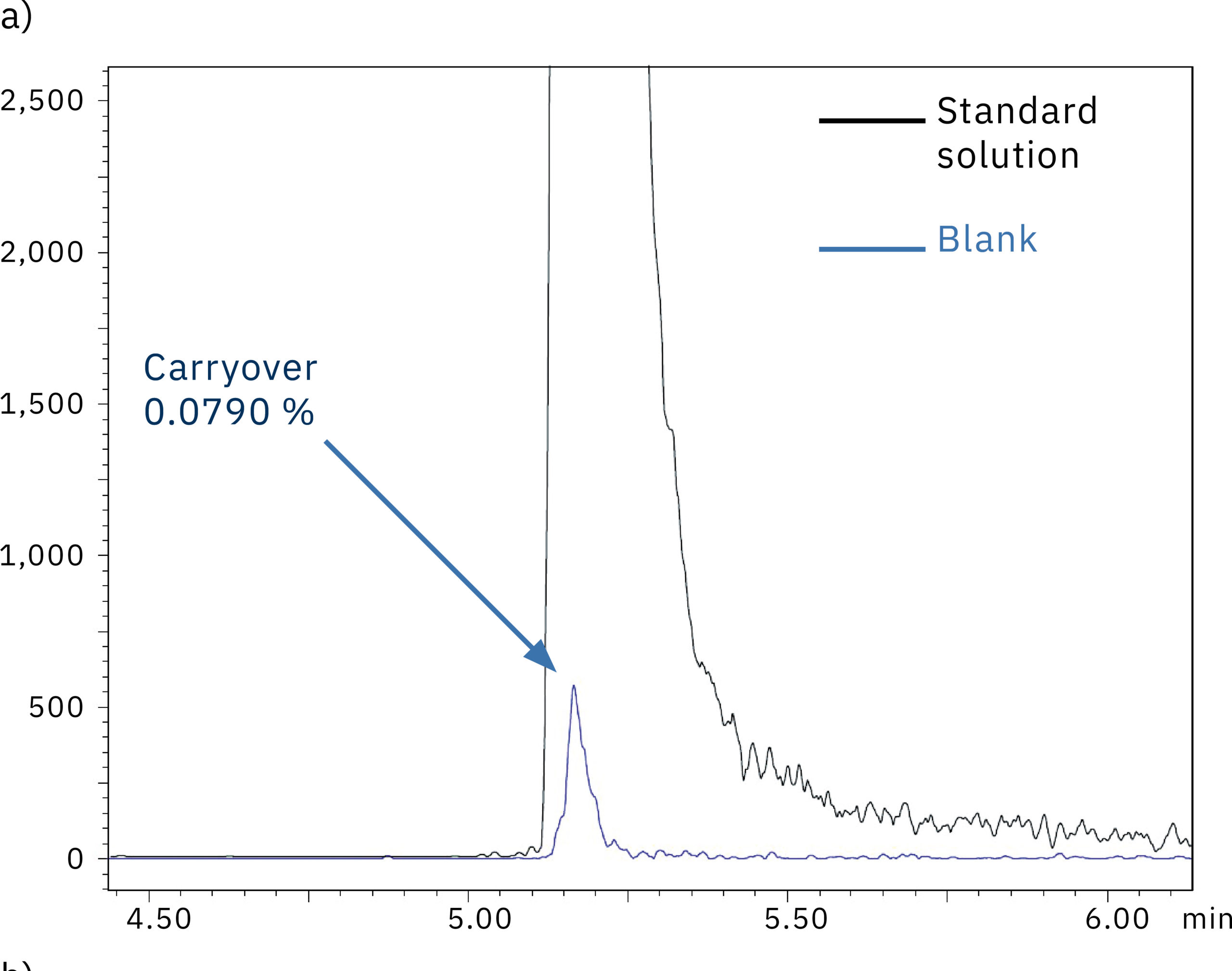
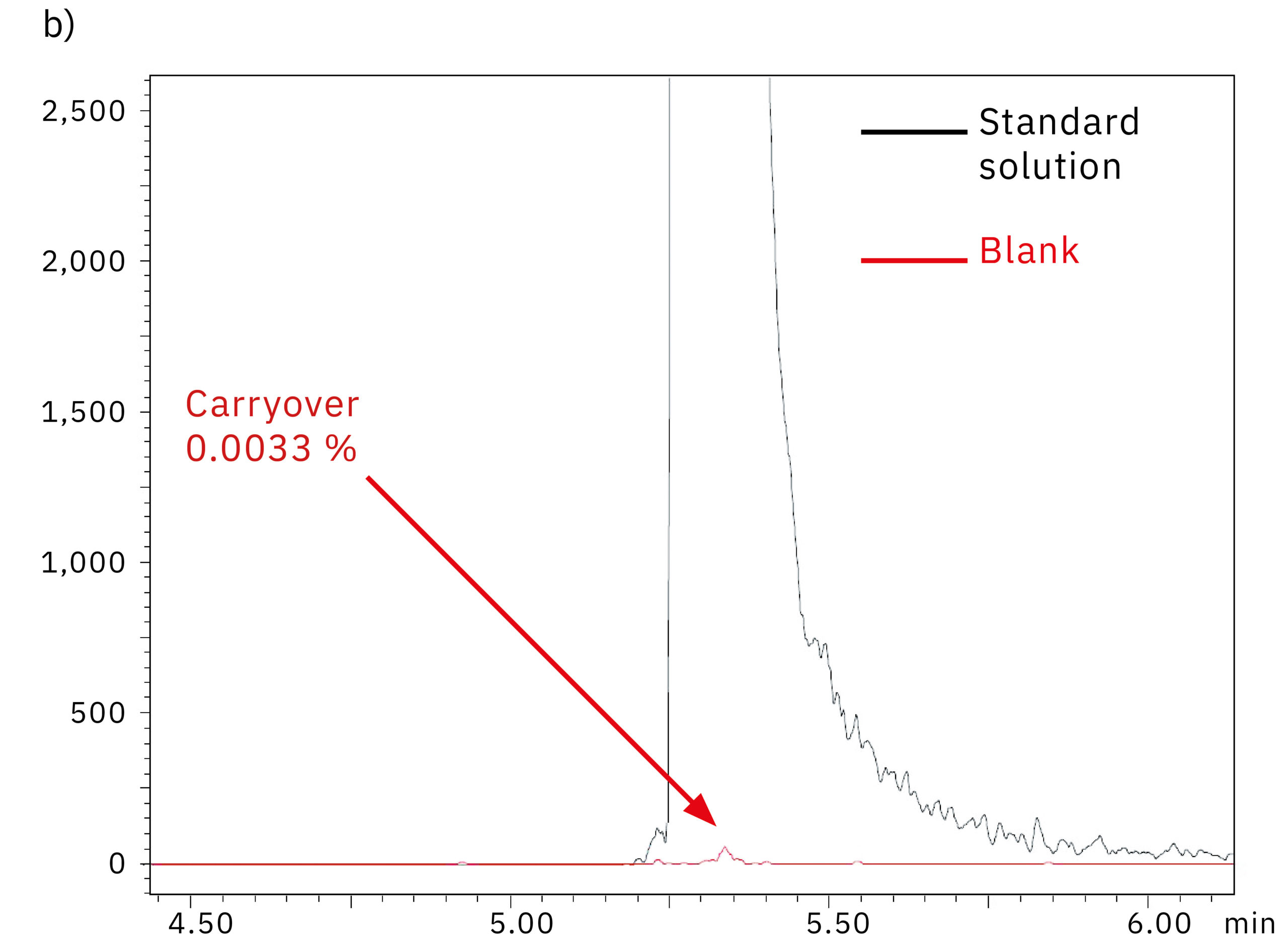
To determine the system performance with regards to carry-over, a blank (water) was injected right after the 1,000 ng/mL oligonucleotide in water standard solution. Figure 5 shows the comparison of the chromatograms obtained from the blank injection on the SUS-based Nexera XR system (a) and the bioinert Nexera XS inert system (b). The results and the carry-over values of 0.079 % and 0.003 % respectively indicate the absence of metal adsorption in the Nexera XS inert system, which also minimizes carry-over into the next sample run.
|
Concentration (ng/mL) |
Nexera XR |
Nexera XS inert |
||
|
ng/mL |
Accuracy |
ng/mL |
Accuracy |
|
|
0.5 |
2.28 |
455.7 |
0.57 |
113.5 |
|
1 |
-1.04 |
-104.4 |
0.93 |
93.0 |
|
5 |
2.43 |
48.5 |
5.42 |
108.5 |
|
10 |
5.62 |
56.2 |
9.13 |
91.3 |
|
50 |
26.63 |
53.3 |
50.24 |
100.6 |
|
100 |
76.39 |
76.4 |
92.77 |
92.8 |
|
500 |
588.74 |
117.7 |
497.04 |
99.4 |
|
1,000 |
965.46 |
96.5 |
1,010.36 |
101.0 |
The comparative evaluation of a stainless-steel-based UHPLC system and a bioinert UHPLC system for the highly sensitive LC-MS analysis of oligonucleotides was carried out with regards to sensitivity, quantitative performance and carry-over. With improvements in all aspects when using the bioinert system, the advantages of removing metal from the sample flow path for the analysis of metal coordinating compounds could be clearly shown. This study emphasizes the value of the Shimadzu Nexera XS inert system in combination with a metal-free separation column for most accurate characterization and quality control of oligonucleotide therapeutics.
Bibliography/Further Information
- C10G-E097 – Oligonucleotide Therapeutics Solution Guide, Shimadzu Corporation, 2022 / First Edition: October 2022
/