Faster throughput. Together.
New streamlined solution for immunosuppressant analysis by LC-MS/MS
Mr. Mikaël Levi, Alsachim / Reagent Kit Business Unit
To face increasing demands on their time and resources, chemical laboratories need easier-to-handle solutions with faster turnaround times, lower costs per sample and full compliance with ever-stricter regulatory standards. Recent work by Alsachim using Shimadzu equipment and software has now led to an optimized high-throughput analytical solution that delivers same-day results for therapeutic drug monitoring of immunosuppressants. The solution combines liquid chromatography and tandem mass spectrometry (LC-MS/MS) with automated sample preparation and a dedicated reagent kit.
Therapeutic drug monitoring (TDM) is a multi-disciplinary science that aims to understand the factors that determine the dose-effect relationship and to use this knowledge to optimize drug treatment (i.e. by maximizing efficacy and minimizing side effects). In many clinical chemistry laboratories, the panels of drugs subject to routine TDM in patients is limited and includes antibiotics, antiepileptics, antidepressants, digoxin, methotrexate and several immunosuppressive drugs. This reflects the need to monitor drug classes with a narrow therapeutic index, established consequences for under- or over-dosing, a defined relationship between concentration in blood and clinical/toxic effect, significant variation within and between individuals and for drugs that have a proven knowledge base for clinical management .[1–3]
In most laboratories, automated immunoassay platforms dominate in TDM. However, these may produce biased results due to the cross reactivity of the active metabolites. This complication may be compounded by batch-to-batch heterogeneity in antibodies or reagent quality, high-dose-hook effects and – for some drug immunoassays – a high cost per analysis.[4]
As precision medicine emerges as an approach to individualized patient treatment – i.e., addressing a specific disease and taking into account individual variability in genes, environment and lifestyle – TDM is likely to have an even greater impact on dose adjustment. Already, this new application for TDM is increasing the number of samples to assay, as well as the pressure for rapid turnaround times. Adding to these challenges is the need to comply with European regulations for in vitro diagnostics (IVDR) that came into force in May 2022 and which have dramatically heightened the need for certified and compliant analytical solutions, including instruments and reagents.
This article highlights a time-saving, complete and fully compliant high-throughput solution for immunosuppressant analysis – created by a combination of liquid chromatography and tandem mass spectrometry (LC-MS/MS) with automated sample preparation and a dedicated reagent kit.
LC-MS/MS for TDM
Liquid chromatography (LC) techniques using ultra-violet (UV), photo diode array (PDA) and fluorescent detection (FLD) systems have been used in TDM for several decades. However, despite the impact of ultra-high-performance liquid chromatography (UHPLC),[5–7] such detection techniques are limited in terms of specificity – often resulting in extensive sample cycle times and poor sensitivity, as many compounds lack natural chromophores or fluorophores.[1]
Mass spectrometry (MS) is now regarded as a key technique for laboratories delivering robust platforms with highly selective and sensitive detection.[8] The capability of MS in the development of assays for individual drugs (“laboratory‐developed tests” – LDTs – or “in‐house” assays) and in multiplexing analyte panels creates new opportunities while expanding the number of drug assays. By increasing the number of drug assays, helping to provide better access to the technology and using novel blood sampling strategies including micro-sampling for pediatric TDM or at-home sampling,[9–12] TDM is also well positioned to support the growing field of personalized medicine.

Automated sample management and preparation
Biological fluids are highly complex matrices which present challenges in matrix management, as endogenous and exogenous components result in compound and system-specific effects in MS. Negating the effects of the matrix must be carefully considered in all sample preparation and management protocols. Matrix effects can lead to isobaric interference, particulate clogging and ion suppression or enhancement, resulting in a difference between the signal intensity detected in a neat standard solution (compared with a matrix-matched standard).[13–16]
In electrospray ionization, ion suppression is due to a change in the droplet formation and surface tension that affects charge-transfer efficiency. Non-volatile compounds such as blood phospholipids, salts, uncharged matrix components, reagent impurities, drugs/metabolites are known to produce ion suppression or enhancement.[17] To help reduce the impact of matrix effects on bioanalytical assays, there are several strategies open to the analyst. One of the most important techniques is to use appropriate and validated internal standards (IS), particularly stable-isotope-labelled analogues (SIL-ISTD), to help correct for ion signal changes and handling errors in sampling preparation protocols. However, for many assays the effect of high inter- and intra-patient variability in endogenous molecule concentrations also requires sample clean-up using extraction or purification techniques such as liquid-liquid extraction (LLE), solid-phase extraction (SPE) or protein precipitation (PPT). Each technique needs to be considered in the context of assay performance in achieving an acceptable level of accuracy and precision, while also paying attention to the efficiency and recovery of the extraction, its ease of use and cost per sample.
For high-throughput assays, the design of the sample preparation method can benefit greatly from automated systems. Robotic liquid handlers with multiple pipettes, such as the Hamilton Microlab Star, can manage simple or complex sample preparation protocols with a high number of samples.
Immunosuppressant drugs
Immunosuppressant drugs are indicated to reduce the activity of the immune system to prevent transplant rejection. The major drugs used are calcineurin inhibitors, cyclosporin and tacrolimus and the mTOR inhibitors, sirolimus and everolimus. Circulating concentrations of these compounds should remain within a narrow therapeutic window, as overdosing can cause serious toxicity and long-term morbidity, while underdosing can cause graft rejection.[3] As immunosuppressant drugs result in a high pharmacokinetic variability between individual patients, TDM is now an established approach to mitigate the risks associated with organ transplantation.
Several commercial immunoassays are available for the TDM of immunosuppressants; however, all immunoassays show a significant positive bias compared to LC-MS/MS methods.[18] Despite the availability of automated immunoassays, each test is restricted to one analyte per test even though multiple immunosuppressants are used with an individual patient in many clinical settings.[1, 19] The following paragraphs describe a new streamlined and automated LC-MS/MS method for the routine TDM analysis of immunosuppressants.
Combining individual solutions
To meet the need for high-throughput analysis, a DOSIMMUNE™ kit (Alsachim) was adapted to robotic liquid handling. This kit, validated and CE-IVD marked with manual sample preparation, provides all the necessary reagents (mobile phases, buffers, stable-isotope-labelled internal standards) and consumables (columns) to monitor immunosuppressants in whole blood by LC-MS/MS. Calibrator and control vials can be directly positioned in robot racks (Figure 1). However, liquid handlers have a larger dead volume that cannot be sucked up, compared to manual pipetting. So, two vials of each calibrator and control level were pooled prior to installation on the racks. With this adjustment, calibrators and controls can be used for an entire week before being replaced.
In order to reduce the number of consumables and protocol steps, an IS solution was mixed directly with the extraction buffer. The mixture can be then dispensed in a reservoir or through a solvent delivery unit.
Workflow optimization of the liquid handler was conducted to reach the shortest possible preparation time, while maintaining the performance obtained with the manual protocol. Most efforts were concentrated on the blood resuspension step. Indeed, whole blood settles under the influence of gravity, leading to plasma and red blood cell separation. To obtain accurate results, samples should be homogeneous during sampling. Therefore, resuspension of each sample was performed just before sampling. A specific protocol was developed using several sequences of aspiration/delivery using the sample height follow-up function of the robot. With this protocol, samples with volumes ranging from 0.5 to 5 mL and haematocrit values from 30 to 70 % were successfully homogenized with a 60 % time reduction compared to similar protocols observed in laboratories. Vortex and centrifugation time/speed parameters were also optimized and operations parallelized as much as possible. Finally, a complete 96-well plate (blank + 6 calibrators, 4 controls and 85 samples) was provided ready-to-be-assayed in 45 minutes. Compared to traditional robotic solutions for immunosuppressant analysis, this corresponds to a 50 % reduction in preparation time.
Immunosuppressants are complex hydrophobic compounds that are known to produce carry-over in LC. Therefore, an efficient cleaning of the injection system is mandatory between samples to provide confidence in result accuracy. The new Nexera X3 LC system (Shimadzu) has improved rinsing capabilities that dramatically reduce the time needed to clean inside the needle. Consequently, the LC-MS/MS cycle time was reduced from 2.1 to 1.5 min (injection-to-injection). When considering the number of samples to assay, this 30 % reduction greatly improves turnaround time.
Evaluation and validation
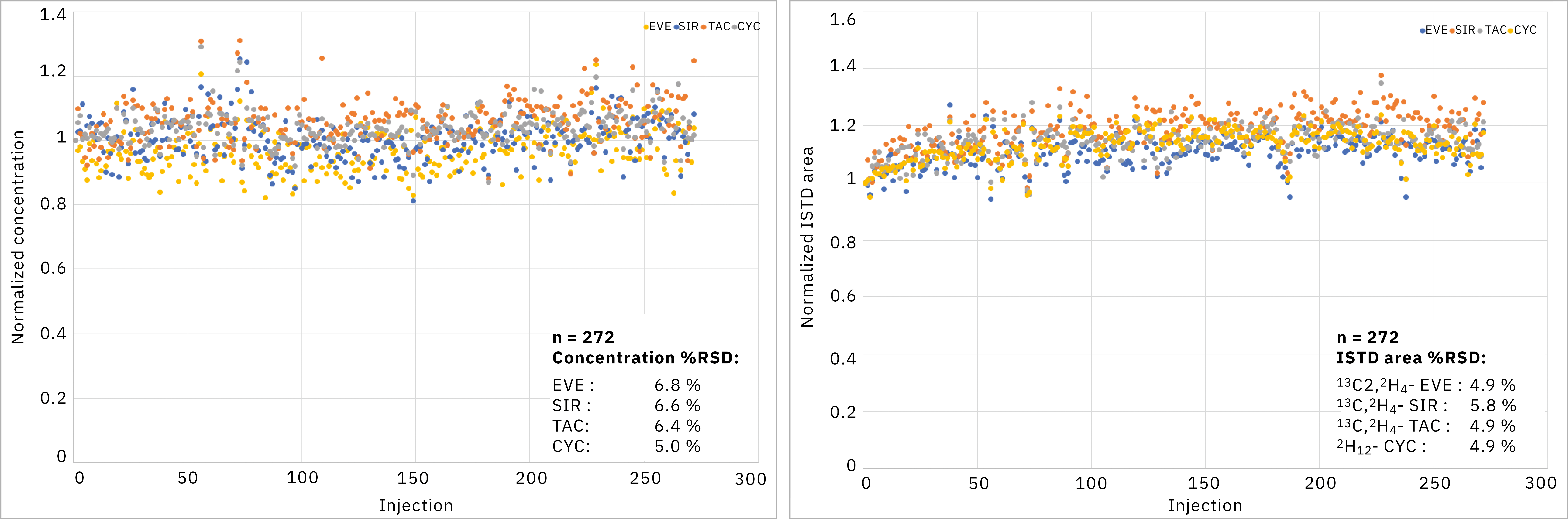
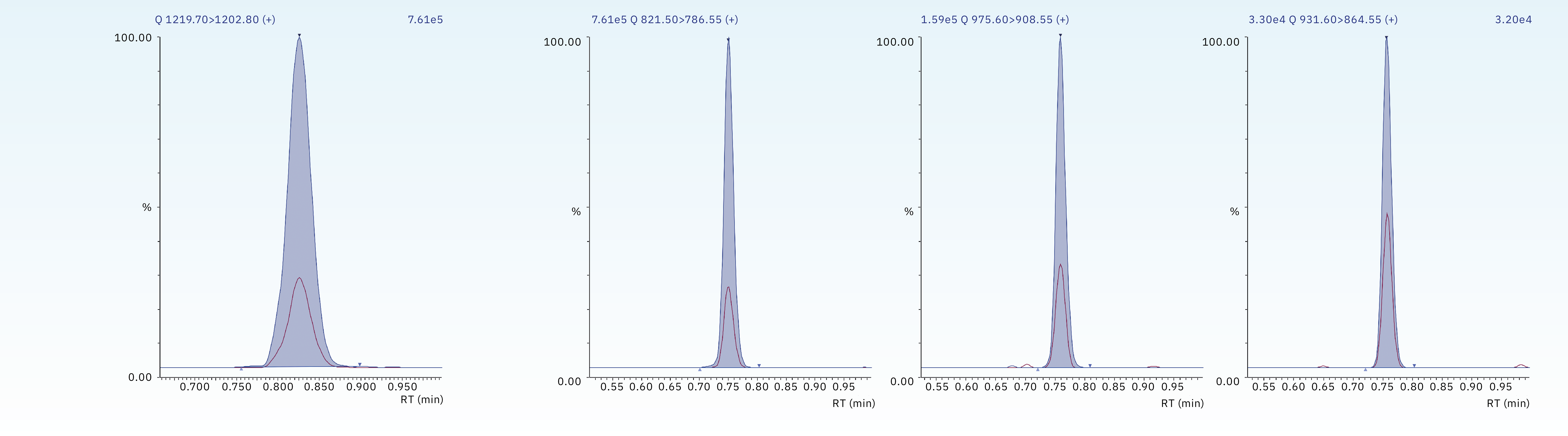
Precision and stability of the solution were evaluated by sequentially preparing three plates containing only the same control at low-mid levels (tacrolimus, sirolimus & everolimus at about 8 µg/L, cyclosporin A at about 145 µg/L) as unknown sample. The first plate included calibrators and controls; other plates included controls. Plates were assayed within the same sequence. Finally, 272 data points were obtained across the three plates. Figure 2 shows the measured concentrations for all compounds and the measured IS peak area. The relative standard deviation of concentrations or peak areas were well below the usually accepted criteria of 15 %. This demonstrated that the MS calibration was stable (with no need to calibrate for each plate), that the sample preparation precision was excellent and reagents were stable on robot tray. A typical chromatogram of such low-mid level control samples is shown in Figure 3.
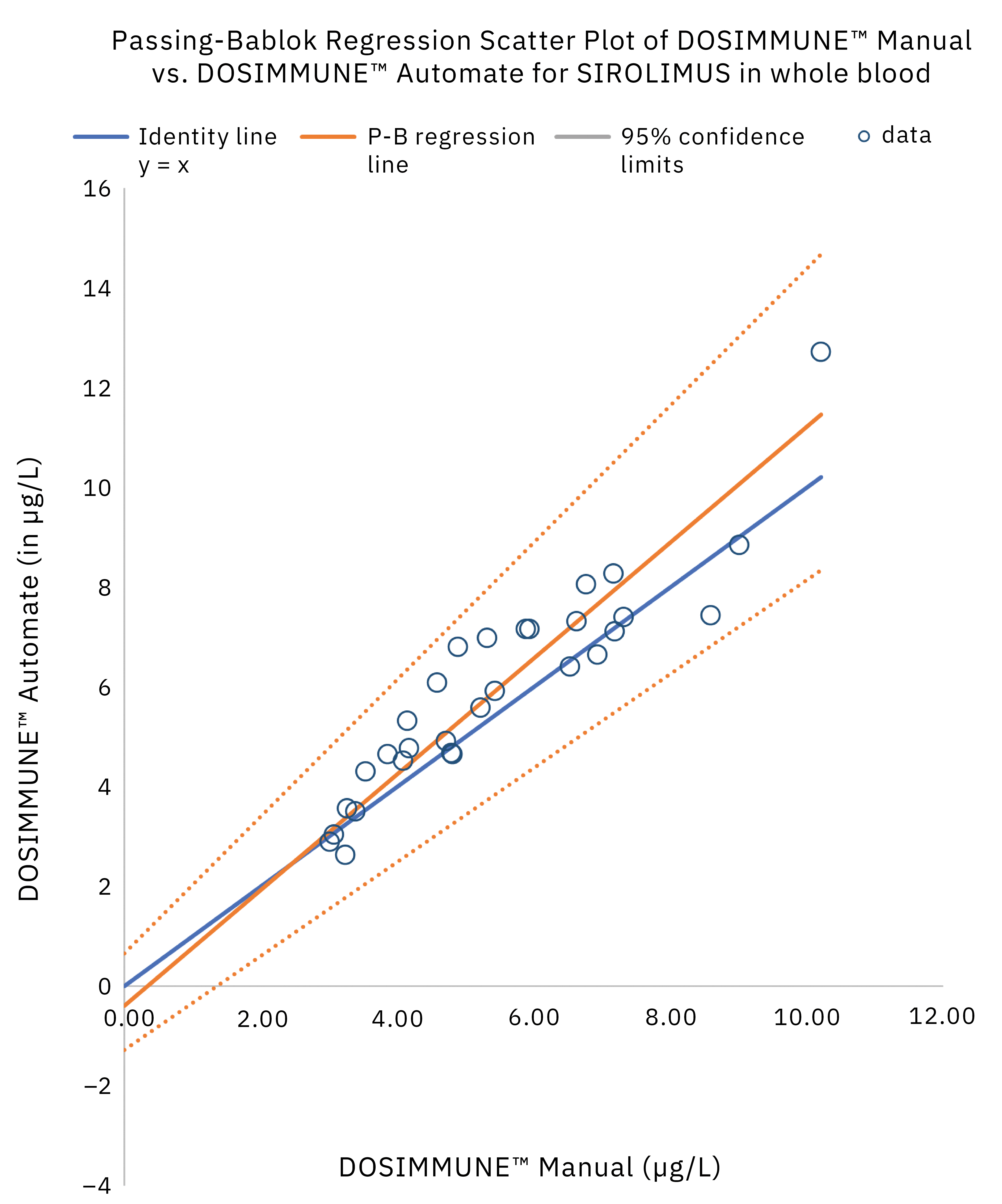
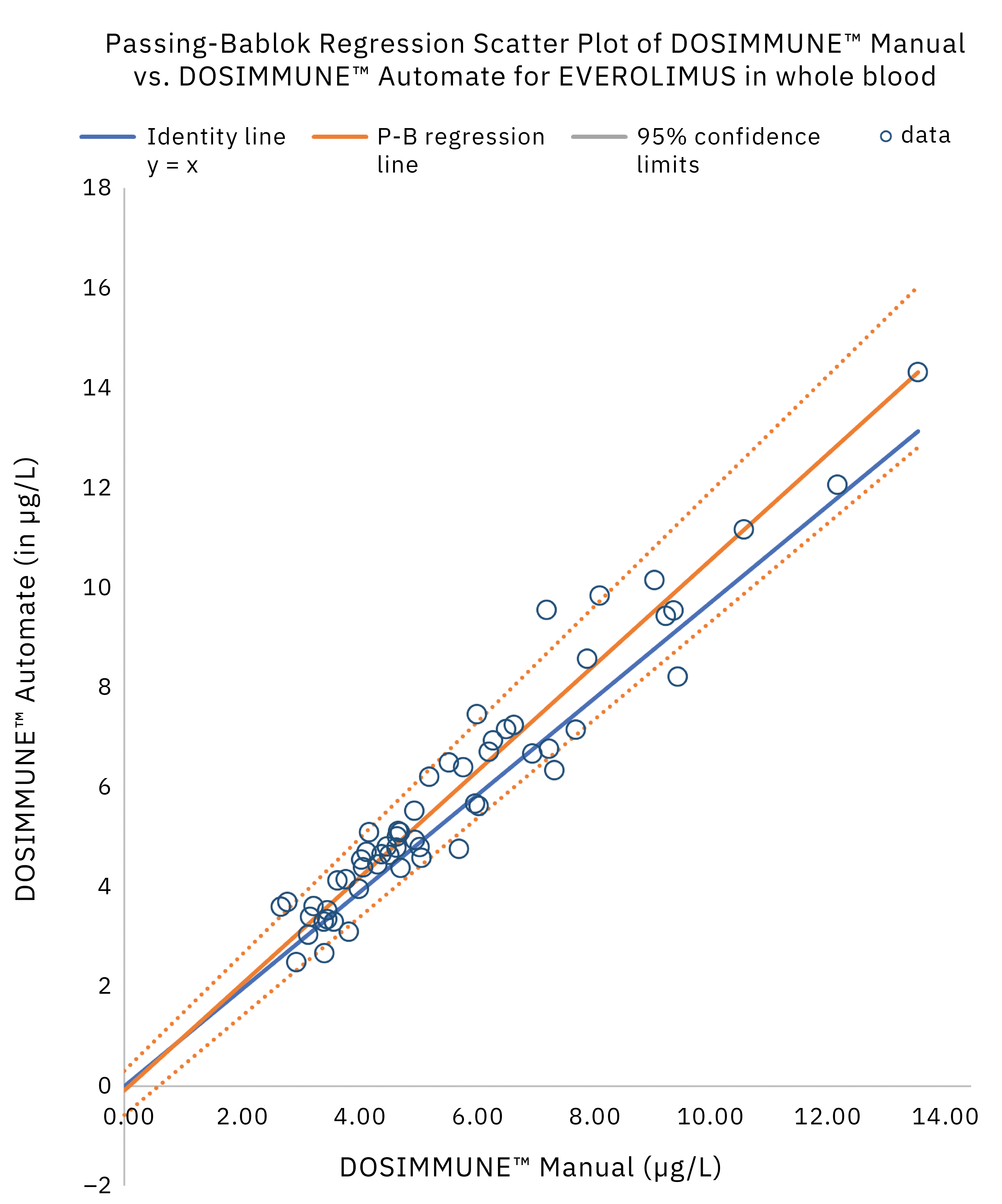
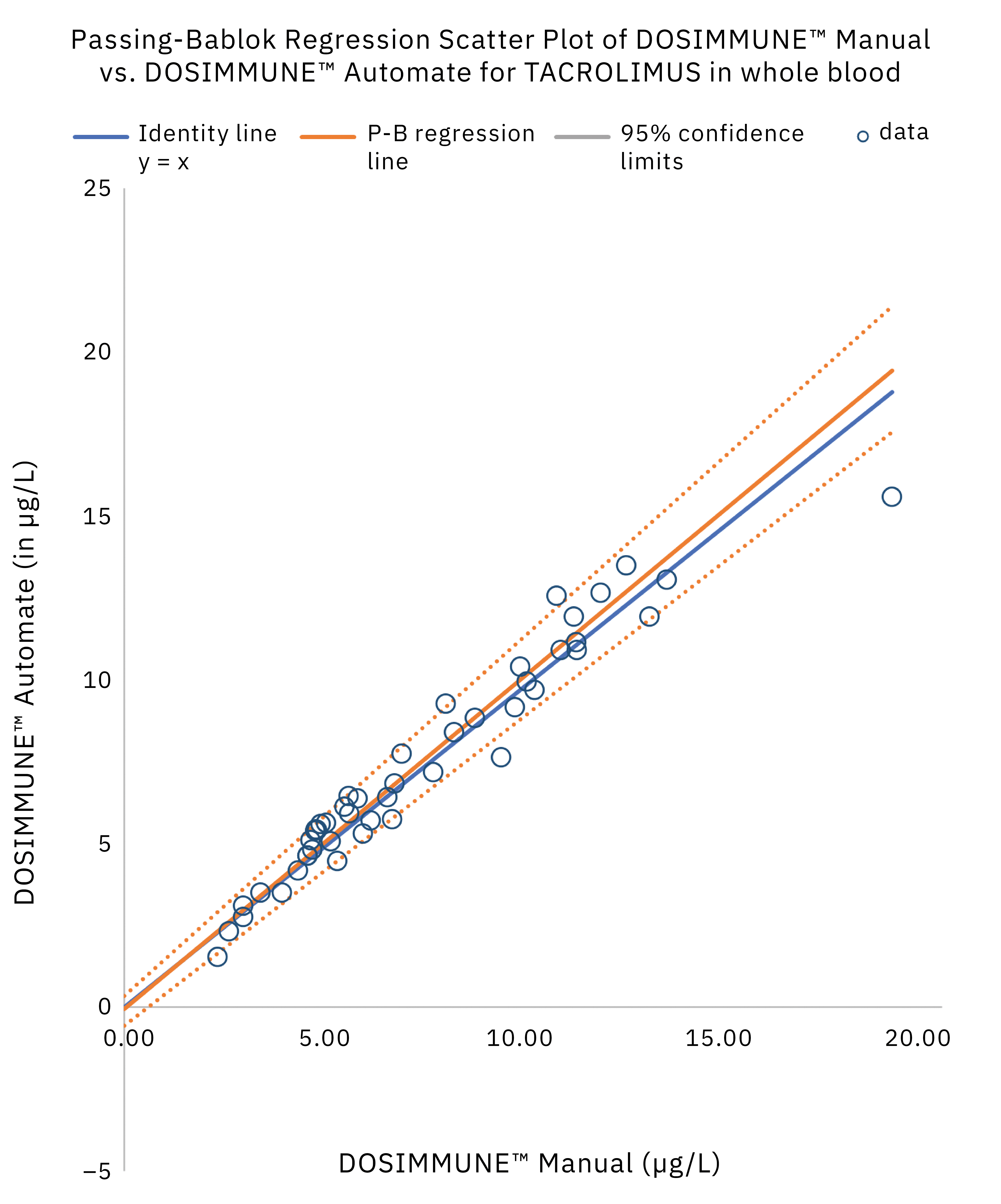
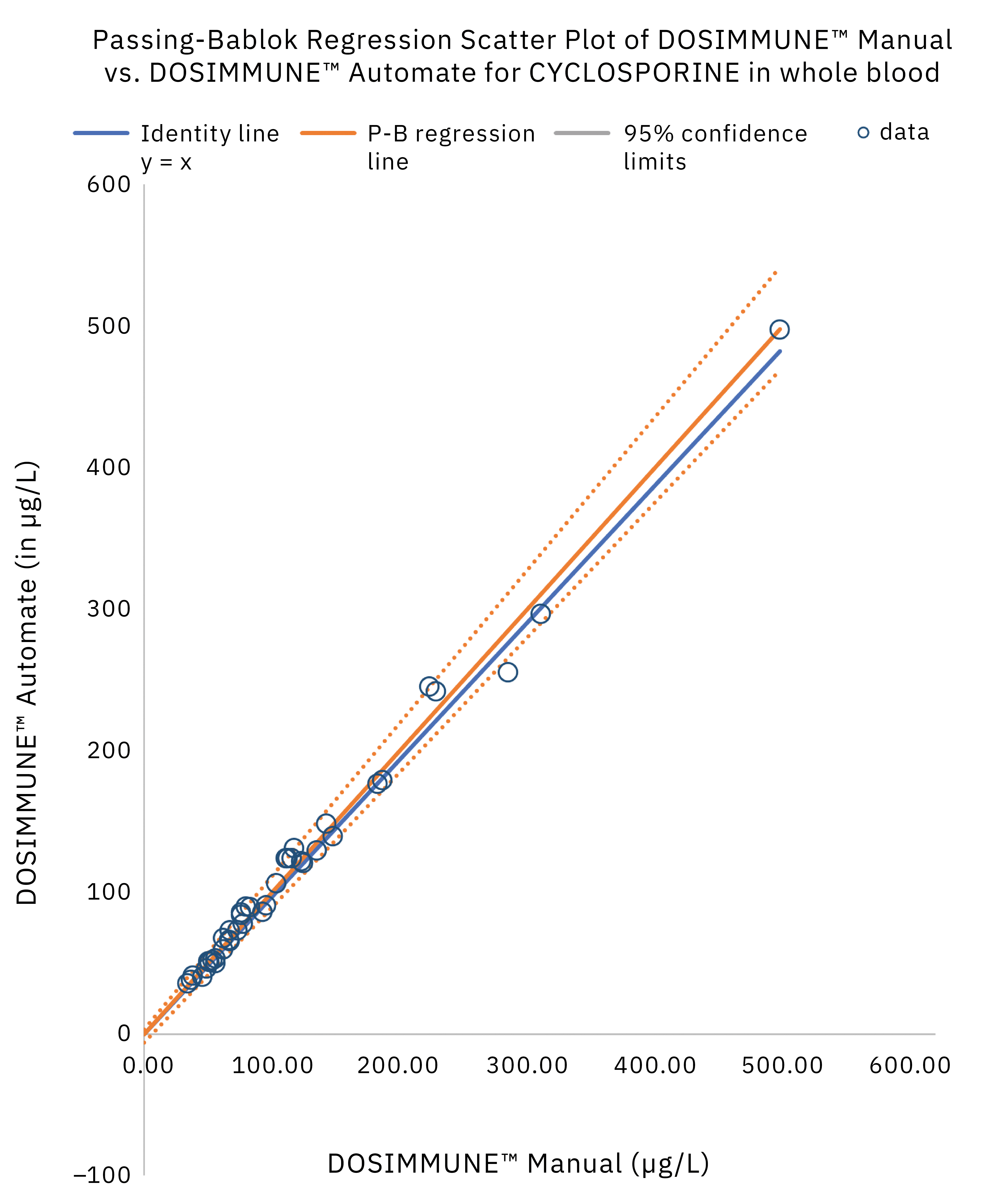
The method including sample preparation was validated according to international guidelines for bioanalytical methods (ICH M10[20], CLSI C62-A[21]). In addition, clinical performance evaluation was evaluated by comparing the results obtained on real patient samples using manual preparation versus the automated protocol. Statistical analysis (Passing-Bablok regression, Bland-Altman plot statistics) demonstrated that the methods were in close agreement and commutable. Figure 4 shows the results of Passing-Bablok regression analysis.
Throughput and compliance
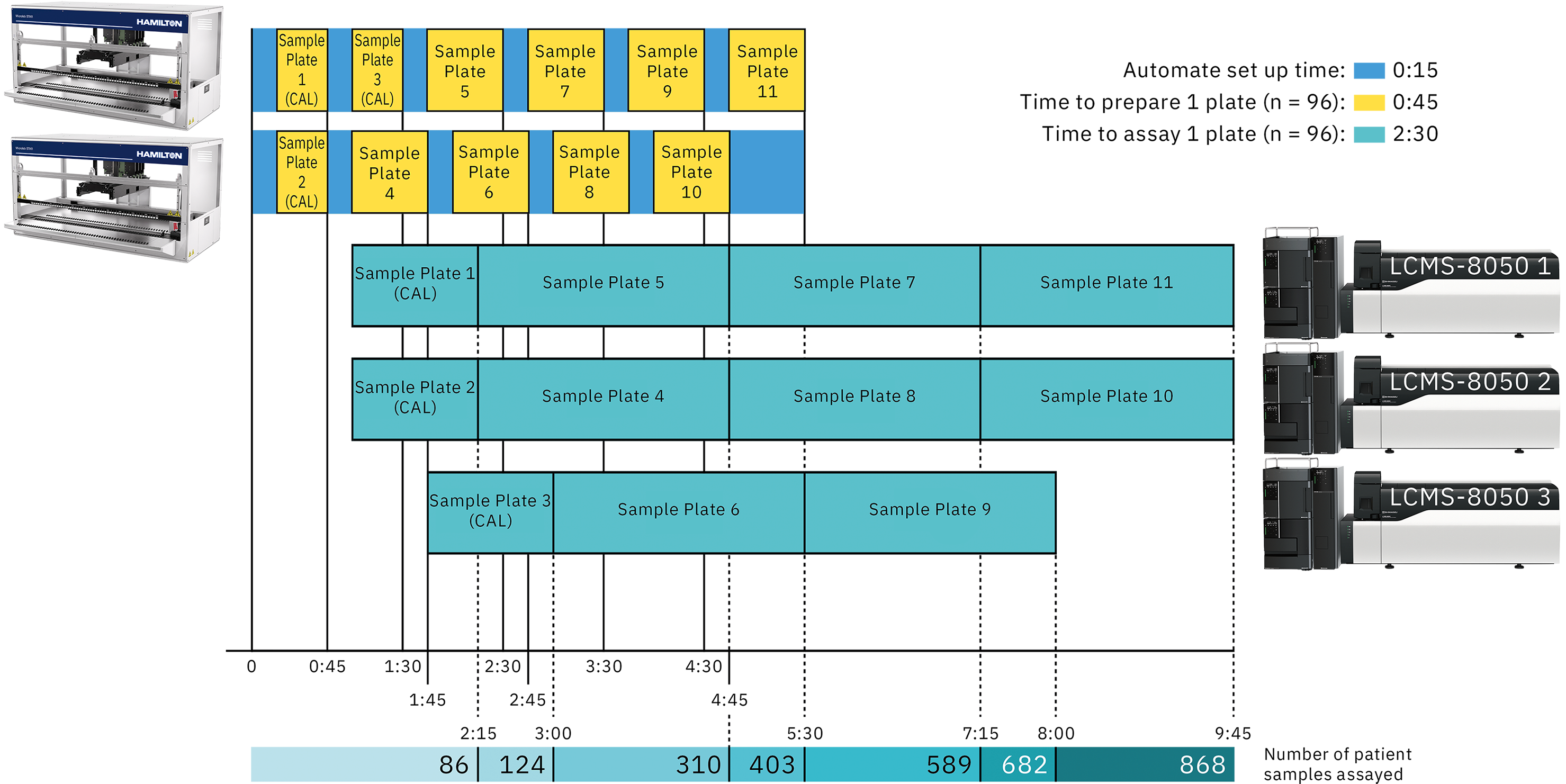
Thanks to the fast sample preparation and the reduced time necessary to assay a full plate, many different configurations can be proposed to fit laboratory throughput. By adjusting the number of liquid handlers and LC-MS/MS systems, it is possible to assay from 300 to almost 900 patient specimens in less than 10 working hours. Figure 5 shows an example of a suggested configuration to reach very high throughput.
In addition to the analytical optimization, informatic connections were studied to be in line with the system speed. The liquid handler automatically generates the working list by scanning the patient tubes and this list is exported directly in the correct format to the Shimadzu LabSolutions software for LC-MS. With a few clicks, the sample assay sequence is created. LabSolutions Insight allows efficient and easy data processing. Using adaptive flagging rules, the software proposes a review-by-exception that highlights the data that need to be looked at by the analyst. Double data validation is possible before sending the results to the laboratory information system through csv files or HL7 bidirectional connection.
All of the instruments and software involved in this time-saving solution are CE-IVD certified. The DOSIMMUNE™ kit has also been certified as CE-IVD with manual or automated sample preparation.
In summary, an efficient and time-saving new method using an optimized and fully compliant combination of instruments, reagents and software now exists for high-throughput analysis of immunosuppressants.
Bibliography/Further Information
- F. Saint-Marcoux, F.-L. Sauvage, P. Marquet. Anal. Bioanal. Chem 388 (2007) 1327–1349.
- J.E. Adaway, B.G. Keevil. J. Chromatogr. B 883–884 (2012) 33–49.
- P.J. Taylor, C.-H. Tai, M.E. Franklin, P.I. Pillans. Clin. Biochem. 44 (2011) 14–20.
- M. Vogeser, C. Seger. J. Chromatogr. B 883–884 (2012) 1–2.
- S. Barco, A. Zunino, A. D’Avolio, L. Barbagallo, A. Maffia, G. Tripodi, E. Castagnola, G. Cangemi. J. Pharm. Biomed. Anal. 138 (2017) 142–145.
- B. Toussaint, F. Lanternier, C. Woloch, D. Fournier, M. Launay, E. Billaud, E. Dannaoui, O. Lortholary, V. Jullien. J. Chromatogr. B 1046 (2017) 26–33.
- V. Mistretta, N. Dubois, R. Denooz, C. Charlier. Acta Clinica Belgica 69 (2014) 53–61.
- M. Himmelbasch. J. Chromatogr. B 883–884 (2012) 3–17.
- B.G. Keevil, J. Fildes, A. Baynes, N. Yonan. Ann. Clin. Biochem 46 (2009) 144–145.
- N. Yonan, R. Martyszczuk, A. Machaal, A. Baynes, B.G. Keevil. Clin. Transplant. 20 (2006) 221–225.
- W. Li, F.L.S. Tse. Biomed. Chromatogr. 24 (2010) 49–65.
- P.M. Edelbroek, J. Van der Heijden, L.M.L. Stolk. Ther. Drug. Monit. 31 (2009) 327–336.
- M. Vogeser, C. Seger. Clin. Chem. 56:8 (2010) 1234–1244.
- T.M. Annesley. Clin. Chem. 49:7 (2003) 1041–1044.
- B.K. Matuszewski, M.L. Constanzer, M. Chavez-Eng. Anal. Chem. 70 (1998) 882–889.
- B.K. Matuszewski, M.L. Constanzer, M. Chavez-Eng. Anal. Chem. 75 (2003) 3019–3030.
- S.T. Wu, D. Schoener, M. Jemal. Rapid. Commun. Mass Spectrom. 22 (2008) 2873–2881.
- W. Tszyrsznic, A. Borowiec, E. Pawlowska, R. Jaswiec, D. Zochowska, I. Bartlomiejczyk, J. Zegarska, L. Paczek, M. Dadlez. J. Chromatogr. B 928 (2013) 9–15.
- F. Aucella, V. Lauriola, G. Vecchione, G.L. Tiscia, E. Grandone. J. Pharm. Biomed. Anal. 86 (2013) 123–126.
- ICH guideline M10 on bioanalytical method validation EMA/CHMP/ICH/172948/2019.
- CLSI. Liquid Chromatography-Mass spectrometry Methods – Approved Guideline. CLSI document C62-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.