High quality analysis, even at low concentration
Evaluating MRM analysis by GC-MS/MS of PAH in palm oil
Waldemar Weber, Shimadzu Europa GmbH, Elvi Horiyanto, Cynthia Lahey, Shimadzu Asia Pacific
Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds primarily formed from the incomplete combustion of organic materials. PAHs are carcinogenic, teratogenic and mutagenic contaminants that are toxic to human health. A major source of PAH exposure is food, in particular edible oils because of their lipophilic nature and high consumption. This article discusses a new multiple-reaction monitoring (MRM) method capable of PAH detection in palm oil, even at low concentration levels.
Polycyclic aromatic hydrocarbons (PAHs) may be introduced into edible oils naturally or through the production drying process. In 2002, the Scientific Committee on Food (SCF) identified 15 PAHs that could be regarded as genotoxic and carcinogenic. In 2005, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) added one PAH to that list, creating what is known as the 15+1 EU Priority PAH.
From this list, European Commission Regulation No 835/2011 stipulates the maximum limit of benzo[a]pyrene and the sum of the PAHs benzo[a]pyrene, benz[a]anthracene, benzo[b]fluoranthene and chrysene in edible oil to be 2.0 μg/kg and 10.0 μg/kg, respectively. This article describes the establishment and evaluation of a new multiple-reaction monitoring (MRM) method for qualitative and quantitative determination of the 15+1 PAHs in palm oil, with applications for other edible oils as well.
Experimental conditions
A triple-quadrupole GCMS-TQ8050 NX was employed in this work. The details of the system and analytical conditions for the GC-MS/MS method are shown in Table 1. Each of the 15+1 PAHs was monitored via quantitative and qualitative MRM transitions. The details of MRM transitions, collision energy (CE) values and internal standard (IS) grouping are tabulated in Table 2.
Standards and sample preparation
The matrix blank was prepared using palm oil purchased from Indonesia. An EU 15+1 PAH standard mixture (100 mg/L) was obtained from Restek Corporation, USA. Chrysene-d12 and perylene-d12 internal standards were obtained from Cambridge Isotope Laboratories Inc., USA. Matrix-matched IS calibration solutions were then prepared accordingly in the matrix blank. The 15+1 PAH concentrations prepared were 0.2, 0.5, 1, 2, 5 and 10 μg/L (which covers 0.44–22.34 μg/kg, subject to the weight of palm oil used). Each of the calibration solutions contained IS concentrations of 5 μg/L chrysene-d12 and 5 μg/L perylene-d12. To determine the recovery, the matrix blank (spiked with the PAH standard mixture and internal standards before extraction) was analyzed.
|
System configuration |
|
|
GCMS system |
GCMS-TQ8050 NX |
|
Liquid sampler |
|
|
Gas chromatography parameters |
|
|
Capillary column |
SH-I-PAH |
|
Injection mode |
Splitless, 330 °C |
|
Flow control mode |
Linear velocity 50.0 cm/s |
|
Carrier gas |
Helium |
|
Temp. program |
110 °C for 1 min, 30 °C/min to 240 °C, |
|
MS parameters |
|
|
Ionization mode |
EI |
|
Ion source temp. |
230 °C |
|
Interface temp. |
300 °C |
|
Mode |
MRM |
|
Analyte |
MRM-1 |
CE (V) |
MRM-2 |
CE (V) |
|
Benzo[c]fluorene |
216.1 > 215.1 |
22 |
216.1 > 189.1 |
30 |
|
Benzo[a]anthracene |
228.1 > 226.1 |
28 |
228.1 > 202.1 |
26 |
|
Chrysene-d12 (IS) |
240.2 > 236.2 |
28 |
240.2 > 238.2 |
26 |
|
Cyclopenta[c,d]pyrene |
226.1 > 224.1 |
38 |
226.1 > 200.1 |
30 |
|
Chrysene |
228.1 > 226.1 |
28 |
228.1 > 202.1 |
26 |
|
5-Methylchrysene |
242.1 > 239.1 |
32 |
242.1 > 215.1 |
22 |
|
Benzo[b]fluoranthene |
252.1 > 250.1 |
28 |
252.1 > 226.1 |
30 |
|
Benzo[j]fluoranthene |
252.1 > 250.1 |
30 |
252.1 > 226.1 |
30 |
|
Benzo[k]fluoranthene |
252.1 > 250.1 |
30 |
252.1 > 226.1 |
30 |
|
Benzo[a]pyrene |
252.1 > 250.1 |
30 |
252.1 > 226.1 |
24 |
|
Perylene-d12 (IS) |
264.2 > 260.2 |
47 |
264.2 > 262.2 |
44 |
|
Indeno[1,2,3-cd]pyrene |
276.1 > 274.1 |
34 |
276.1 > 250.1 |
30 |
|
Dibenzo[a,h]anthracene |
278.1 > 276.1 |
30 |
278.1 > 252.1 |
30 |
|
Benzo[g,h,i]perylene |
276.1 > 274.1 |
32 |
276.1 > 275.1 |
28 |
|
Dibenzo[a,l]pyrene |
302.1 > 300.1 |
36 |
302.1 > 298.1 |
60 |
|
Dibenzo[a,e]pyrene |
302.1 > 300.1 |
36 |
302.1 > 276.1 |
28 |
|
Dibenzo[a,i]pyrene |
302.1 > 300.1 |
36 |
302.1 > 276.1 |
28 |
|
Dibenzo[a,h]pyrene |
302.1 > 300.1 |
36 |
302.1 > 276.1 |
28 |
Detection and separation
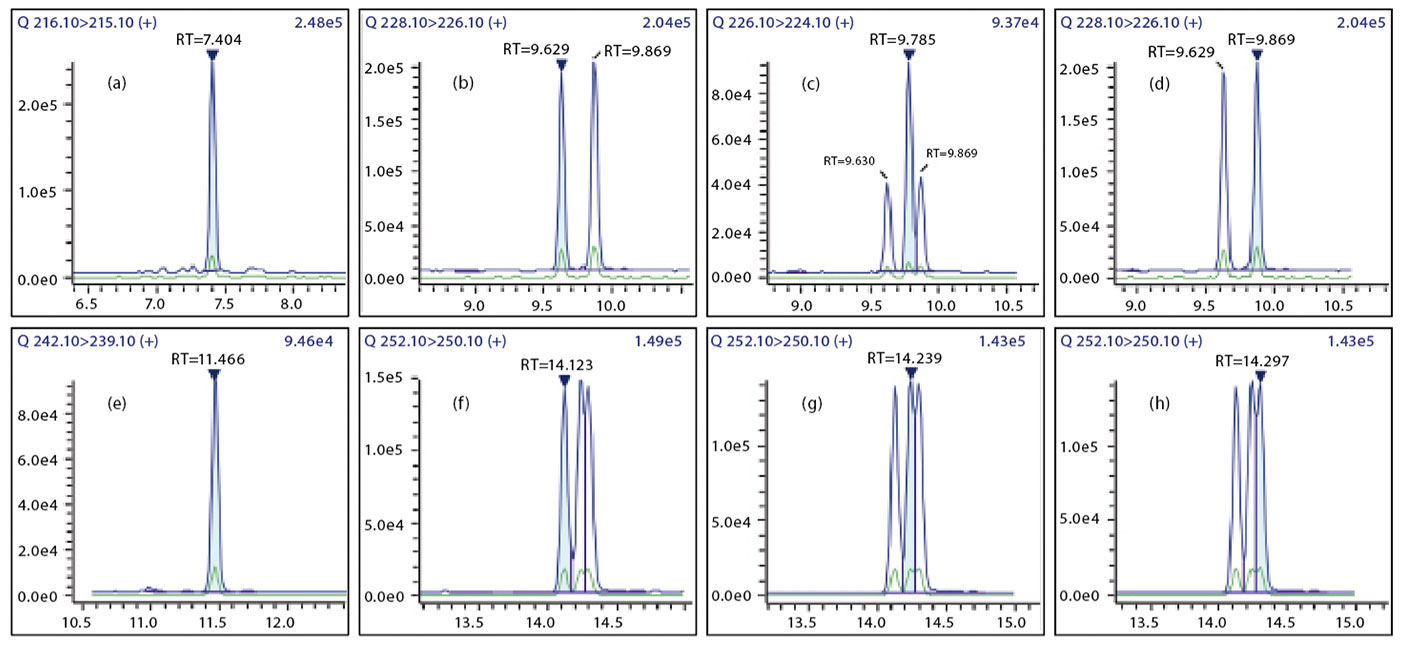
The 15+1 PAHs were separated in the GC-MS/MS using an SH-I-PAH column. The mass chromatograms of all compounds are displayed in Figure 1.
Calibration range, linearity and quantitation
Matrix-matched IS calibration curves were set up for the 15+1 PAHs using prepared calibration solutions. The calibration range of each PAH is shown in Table 3. Calibration curves of all PAHs demonstrated excellent linearity, with R2 value of at least 0.9979 (Table 3). Quantitation of the palm oil sample was done by analyzing the palm oil matrix blank that was spiked with internal standards. None of the 15+1 PAHs was detected in this palm oil sample.
|
Analyte |
Calibration Range µg/L |
R2 |
Conc. spiked for recovery test µg/kg |
Recovery % |
|
Benzo[c]fluorene |
1.0–10 |
0.9994 |
6.53 |
58 |
|
Benzo[a]anthracene |
0.5–10 |
0.9995 |
8.50 |
76 |
|
Cyclopenta[c,d]pyrene |
0.5–10 |
0.9997 |
10.32 |
92 |
|
Cyclopenta[c,d]pyrene |
0.5–10 |
0.9997 |
10.32 |
92 |
|
Chrysene |
0.5–10 |
0.9985 |
9.84 |
88 |
|
5-Methylchrysene |
0.2–10 |
0.9993 |
9.12 |
82 |
|
Benzo[b]fluoranthene |
0.2–10 |
0.9991 |
8.98 |
80 |
|
Benzo[j]fluoranthene |
0.2–10 |
0.9987 |
8.26 |
74 |
|
Benzo[k]fluoranthene |
0.2–10 |
0.9985 |
9.48 |
85 |
|
Benzo[a]pyrene |
0.2–10 |
0.9989 |
9.35 |
84 |
|
Indeno[1,2,3-cd]pyrene |
0.2–10 |
0.9988 |
9.44 |
85 |
|
Dibenzo[a,h]anthracene |
0.2–10 |
0.9990 |
9.49 |
85 |
|
Benzo[g,h,i]perylene |
0.2–10 |
0.9991 |
9.45 |
85 |
|
Dibenzo[a,l]pyrene |
0.2–10 |
0.9986 |
9.01 |
81 |
|
Dibenzo[a,e]pyrene |
0.2–10 |
0.9981 |
9.64 |
86 |
|
Dibenzo[a,i]pyrene |
0.2–10 |
0.9979 |
9.85 |
88 |
|
Dibenzo[a,h]pyrene |
0.5–10 |
0.9988 |
9.25 |
83 |
For recovery calculation, the matrix blank was spiked with 5 μg/L (equivalent to 11.173 μg/kg in palm oil) of both internal standards and PAHs. Concentrations were then quantified using the IS calibration curves. The recovery of so-called PAH4s (benzo[a]pyrene, benz[a]anthracene, benzo[b]fluoranthene and chrysene) was between 76.15 %–88.11 %, which is within the criteria determined by EU Regulation No 836/2011 (50 %–120 %). The quantitation and recovery results of palm oil spiked with the 15+1 PAHs are presented in Table 3. It is worth noting that triphenylene, which shares the same MRM target transition as chrysene, could be an interference during quantification of chrysene. The confirmation method with a different column is recommended to verify the quantitation when chrysene concentration falls above the regulated upper limit.
LOD, LOQ and repeatability
The limit of detection (LOD) of all 15+1 PAHs in the palm oil sample was determined to be in the range of 0.038–0.327 μg/kg; for the PAH4s in the range of 0.071–0.157 μg/kg. In addition, the calculated limit of quantification (LOQ) for the 15+1 PAHs was in the range of 0.127–1.090 µg/kg; for the PAH4s 0.236–0.522 µg/kg. According to European Commission Regulation No 836/2011, the LOD for PAH4s should be < 0.30 µg/kg, with an LOQ < 0.9 µg/kg. Therefore, the GC-MS/MS method used here demonstrates the capability of MRM detection in quantifying even low concentrations of PAH in palm oil.
Analysis method precision was determined by analyzing eight consecutive runs of the low concentration (0.5 μg/L) and mid concentration (5 μg/L) of calibration levels in the matrix. The %RSD of peak area ratio of all PAHs with respect to their IS were < 8 % and < 5 % for low and mid concentration levels, respectively. For greater insight, the 5 μg/L calibration solution in the matrix was analyzed another 72 times within a 48-hour period. The %RSD of peak area ratio of all PAHs (72) with respect to their IS were < 5 % (except for dibenzo[a,i]pyrene which was < 8 %). These results indicate that the optimized MRM method has high stability and analysis precision. The values discussed above are summarized in Table 4.
|
Analyte |
Calculated LOD [µg/kg] |
Calculated LOQ [µg/kg] |
Area ratio %RSD of 0.5 µg/L solution (n=8) |
Area ratio %RSD of 5 µg/L solution (n=8) |
Area ratio %RSD of 5 µg/L solution (n=72) |
|
Benzo[c]fluorene |
0.327 |
1.090 |
6.2 |
1.5 |
2.2 |
|
Benzo[a]anthracene |
0.176 |
0.588 |
5.3 |
1.6 |
2.4 |
|
Cyclopenta[c,d]pyrene |
0.157 |
0.522 |
3.1 |
1.5 |
4.2 |
|
Chrysene |
0.175 |
0.583 |
5.9 |
2.0 |
3.2 |
|
5-Methylchrysene |
0.112 |
0.373 |
2.8 |
1.6 |
2.4 |
|
Benzo[b]fluoranthene |
0.088 |
0.293 |
3.0 |
1.8 |
2.7 |
|
Benzo[j]fluoranthene |
0.077 |
0.256 |
5.2 |
4.1 |
4.3 |
|
Benzo[k]fluoranthene |
0.077 |
0.255 |
5.1 |
3.9 |
4.8 |
|
Benzo[a]pyrene |
0.071 |
0.236 |
4.0 |
2.1 |
2.8 |
|
Indeno[1,2,3-cd]pyrene |
0.049 |
0.163 |
1.4 |
2.2 |
2.3 |
|
Dibenzo[a,h]anthracene |
0.045 |
0.150 |
1.9 |
1.7 |
2.6 |
|
Benzo[g,h,i]perylene |
0.038 |
0.127 |
2.7 |
2.2 |
2.5 |
|
Dibenzo[a,l]pyrene |
0.107 |
0.358 |
7.3 |
3.4 |
4.4 |
|
Dibenzo[a,e]pyrene |
0.127 |
0.423 |
7.2 |
2.4 |
3.7 |
|
Dibenzo[a,i]pyrene |
0.114 |
0.378 |
5.3 |
3.0 |
7.9 |
|
Dibenzo[a,h]pyrene |
0.137 |
0.458 |
5.3 |
3.0 |
4.3 |
An attractive new method
Clearly, this shows the successful development of an MRM method using GCMS-TQ8050 NX for the analysis of polycyclic aromatic hydrocarbons in palm oil, which is suitable for other kinds of edible oils as well. The results show that this method allows the detection and quantification of the 15+1 PAHs even at low concentration: down to 0.071 µg/kg LOD and 0.127 µg/kg LOQ, respectively. Excellent linearity of IS calibration curves with R2 values was also obtained. Finally, in addition to sensitivity and selectivity, the optimized MRM method also showed good repeatability and robustness – making this new method highly attractive for analytical use by laboratories.