Harnessing the power of light
The mighty role of tiny optics in the discovery of our world
Tomofumi Seto, Shimadzu Europa GmbH
The universe, a vast expanse of celestial wonder, has captured the imagination of mankind for centuries. But how do we unravel the mysteries hidden within this cosmic fabric? One of the answers is light. By analyzing the light emitted or absorbed by celestial objects, scientists can reveal a great deal about their composition, temperature, motion and even their distant history. Through the spectroscopy, we can explore the universe in ways never imagined before. This article takes a journey into the field of the spectroscopic elements, “diffraction gratings” offered by Shimadzu and their key role in exploring our world.
The origins of Spectroscopy
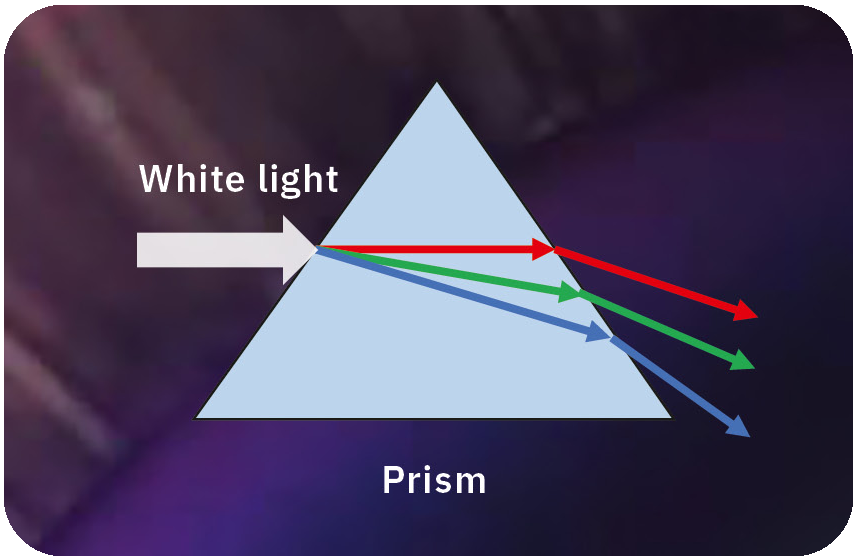
In the 17th century, British physicist Isaac Newton, who made great research achievements in various fields, discovered a secret of light.Using polished transparent devices called prisms, he observed white light split into a band of different colors like a rainbow. His experiments revealed that the white light is composed of different colors, or wavelengths. This means that the sunlight or fluorescent light we usually see includes all wavelengths of light, such as red, green, blue and many more.
A band of different wavelengths discovered by Newton is known as a spectrum. By analyzing the interaction of a material with the spectrum, valuable information about its properties can be obtained. This principle is the basis of modern spectroscopy.


Key device for spectroscopy
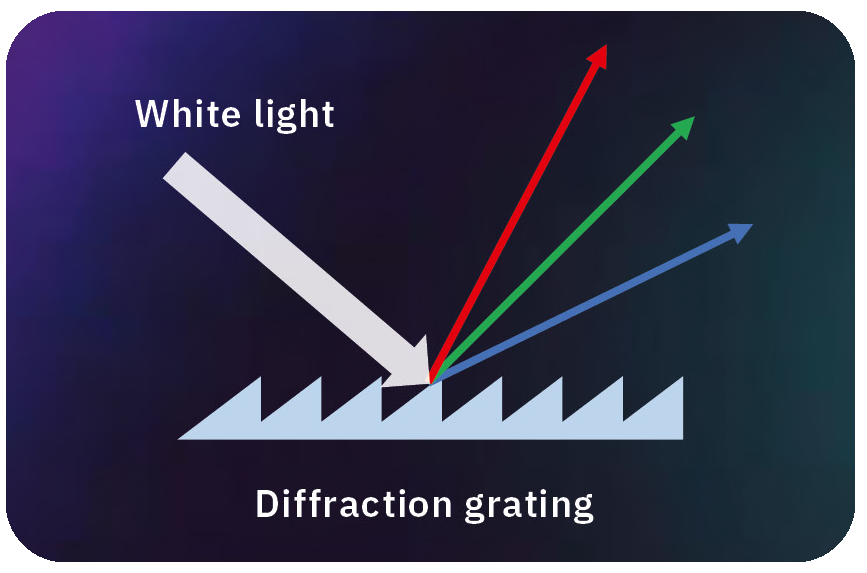
The first step in performing spectroscopy is to use an optical element to split the light into different wavelengths. There are basically two typical spectroscopic elements, prisms and diffraction gratings (Figure 2).
Prisms work on the principle of refraction – they are typically triangular solids made of quartz or glass that bend the angle of light depending on its wavelength due to the different speed of propagation in different media, this property being called the refractive index.
Diffraction gratings use a different principle – diffraction based on the Huygens’ principle, where light is bent around the corners of an obstacle. Therefore, diffraction gratings have countless very small grooves (obstacles) on a substrate surface. Again, the bending angle depends on the wavelength, and this is how different colors can be separated. This effect is what makes the back of a CD or DVD reflect many different colors when viewed from certain angles.
Nowadays, diffraction gratings are the standard for spectroscopic applications because of their optical advantages. The wavelength resolution accuracy is high, and this wavelength accuracy is more stable with temperature changes compared to prisms. Therefore, diffraction gratings are often a more practical spectroscopic choice.
History of diffraction grating
Until the middle of the 20th century, a prism was the main element used in spectroscopy. Diffraction gratings also existed but weren’t widely used because they are difficult to produce. Their grooves should be precisely and uniformly on the surface, but the fabrication technology was not advanced, and therefore their performance was inferior to that of prisms at that time. Additionally, the production number of gratings was limited, and the availability of gratings was inferior in terms of price and time.
However, the manufacturing technology of diffraction gratings was steadily improving. Eventually, with the establishment of high-performance and large-scale production technology of diffraction gratings, they became increasingly popular in spectroscopic instruments in place of prisms.
Shimadzu’s contribution to manufacturing technology of diffraction gratings
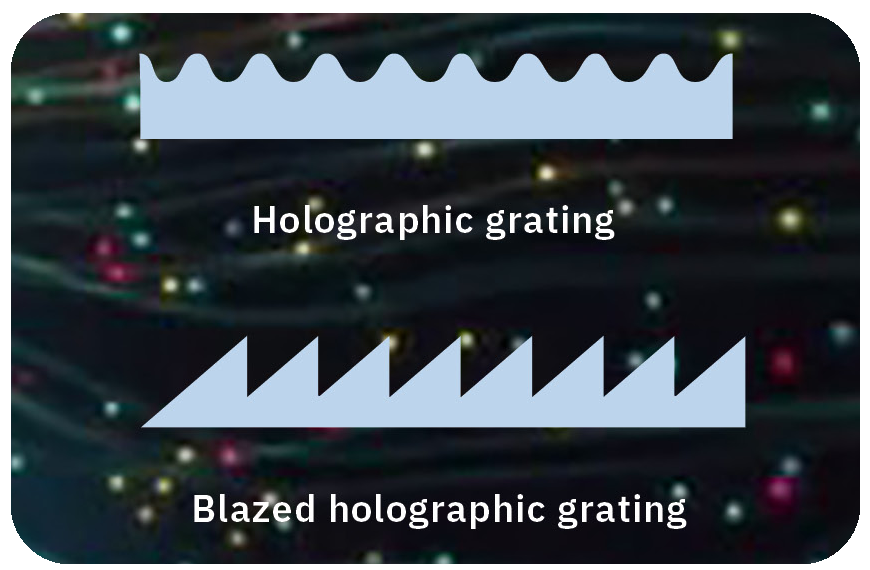
There are typically two types of diffraction gratings. One is a ruled grating, where the grooves are physically engraved by machine. The other is a holographic grating, which is produced by a holographic exposure method using the two-flux interference of a laser.
The holographic grating has the advantage of low stray light. Stray light is generally undesired light or wavelengths that are generated unexpectedly. Especially in the field of spectroscopic analysis, stray light has a negative impact on analysis accuracy. The grooves of a holographic grating are formed more precisely than those of ruled grating so that stray light is lower.
On the other hand, holographic gratings have the disadvantage that the intensity of diffracted light is lower. One way to circumvent this is to use “blazed grooves”, which are saw-shaped grooves that increase the light intensity of desired wavelengths, but in the past these groove shapes could only be manufactured by the ruled method. At that time, it was considered impossible to produce blaze grooves by using the holographic method.
However, Shimadzu was striving to develop a blazed holographic grating which realizes low stray light and the same level of diffraction light intensity as ruled gratings. After many years of joint research with RIKEN (Institute of Physical and Chemical Research), the development of blazing grooves for holographic gratings succeeded and the world’s first manufacturing technology for blazed holographic gratings was established.
Current state of Shimadzu’s diffraction gratings
Nowadays, diffraction gratings have been widely used in several fields with its manufacturing technology and the extensive lineup/custom-made capability according to each application.
In analysis and inspection fields, diffraction gratings contribute to in-house products and external customers as a key device of equipment. Furthermore, the use has been spreading in areas such as food, semiconductor manufacturing and blood testing.
The use of diffraction gratings is not only for analysis purposes but also to support optical communications as a part of wavelength selective switches (WSS). WSS control the wavelength of optical signals to realize efficient transmission of information at optical communications.
Shimadzu’s contribution is not limited to the earth but extends even into space. The spectroscopic satellite “Hisaki” (SPRINT-A) was successfully launched in Japan in 2013. The company’s diffraction grating is used as the heart of the satellite. This satellite is the world’s first space telescope for remote observation of the planets such as Venus, Mars and Jupiter. It analyzes the atmosphere of planets by observing extreme ultraviolet light, which cannot be detected on Earth, and contributes to the elucidation of mysteries in space.
Suitable for each customer’s needs
Shimadzu’s diffraction gratings have been supporting spectroscopic technology in very diverse industrial fields and research institutes and have accumulated technological expertise over many years. Today there are several types of diffraction gratings that are suitable for each customer’s needs and applications, with the advantage of low stray light and high diffraction efficiency. The company’s relentless challenge to meet the diversifying needs of spectroscopy will continue, just like the discovery of the universe.
[1] T. Namioka, J. Spectroscopic. Soc. Japan Vol.34 No.1, 41-53, 1984
[2] M. Seya, J. Spectroscopic. Soc. Japan Vol.19 No.1, 43-51, 1970
[3] K. Sano, Y. Aoyagi, and S. Namba, OYO BUTURI, Vol.48, 539-544, 1979
[4] K. Sano, Y. Aoyagi, and S. Namba, Opt. Commun., Vol.29, 253-255, 1979