Ultra-trace analysis of heavy metals using ICP-MS
Sensitive and robust wastewater analysis to safeguard our waters
Nico Gilles, Shimadzu Deutschland GmbH

Clean water is our most precious resource – vital for a thriving environment, our food and our health. It only makes sense, then, that wastewater is analyzed with the utmost care, especially when it involves toxic substances like heavy metals. As permissible limits are lowered, the requirements for testing laboratories are increasing. More sensitive measurement methods are the key to success here. ICP-MS technology provides exceptional precision for detecting arsenic, lead, cadmium, mercury and other elements – even in trace amounts.
German Waste Water Ordinance: Setting the bar high – even as permissible limits are lowered
The German Waste Water Ordinance (AbwV) aims to preserve the quality of natural waters and promote sustainable water management. It defines specific discharge limits that depend on both the type of water body (such as surface or coastal waters) and the type of wastewater (whether municipal or industrial). Compliance with these limits is crucial when it comes to safeguarding water bodies from substances that can be harmful, such as heavy metals, organic compounds and nutrients.
Before it is discharged, wastewater must be treated mechanically, biologically and, if necessary, chemically. Municipal wastewater treatment plants play a central role in this, since they handle wastewater from private households. There are also extensive monitoring and documentation obligations: Operators are required to regularly measure pollutant concentrations, document the results and submit them to authorities when necessary. In this way, operators can not only ensure compliance with limits but also identify and address operational problems early on. Industrial wastewater is often subject to stricter requirements because it typically contains higher concentrations of pollutants. This guarantees that the established limits are met there as well.
Wastewater analysis – correct and critical
Chemical analysis of wastewater is critical when it comes to ensuring compliance with legal regulations and minimizing the impact of pollutants on ecosystems and human health. The German Environment Agency (UBA) reports that around 3.7 billion cubic meters of wastewater are treated in treatment plants in Germany each year. Harmful substances in wastewater can be detected thanks to chemical analysis. These include heavy metals such as lead, mercury and cadmium, as well as organic contaminants like pesticides and pharmaceutical residues, and nutrients such as dissolved nitrogen and phosphorus compounds. Information on pollutant concentrations helps detect potential threats to the environment, our drinking water and human health, and supports taking suitable action. Given the challenges posed by pollution and climate change, the role of chemical analysis in wastewater treatment and monitoring has never been more crucial for safeguarding our essential water resources sustainably.
The challenge of choice in elemental analysis
For decades, atomic absorption spectroscopy (AAS) and inductively coupled plasma optical emission spectroscopy (ICP-OES) have been well established in elemental and heavy metal analysis. Their advantages – high matrix tolerance, sufficient sensitivity and ease of use – remain central to the analytical techniques used. With the inductively coupled plasma mass spectrometry (ICP-MS) techniques, such as the ICPMS-2040/-2050 instruments, wastewater analysis now has a new option that provides an approved method for measuring the majority of heavy metals regulated under the Waste Water Ordinance. The misconception that ICP-MS is only suitable for drinking water and “clean” samples has long been disproven. Thanks to its exceptional sensitivity and ever-improving matrix tolerance, this technique is being used in a wide range of applications.


Inductively coupled plasma mass spectrometry
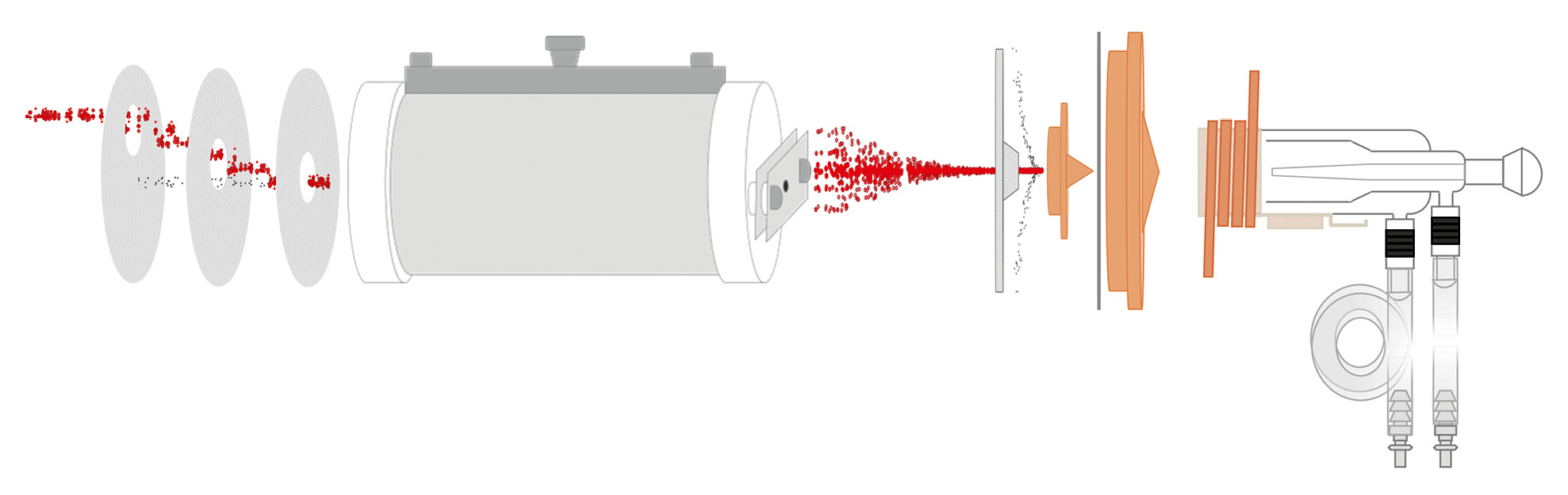
ICP-MS (Inductively Coupled Plasma Mass Spectrometry) is a highly advanced analytical technique used for precisely measuring the concentrations of elements across a wide range of samples. Its main advantage is that it can detect extremely low concentrations of elements, often in the range of a few parts per billion (ppb) or even parts per trillion (ppt).
The way ICP-MS works can be explained in a series of steps. First, the sample, typically a liquid, is carefully prepared through processes such as digestion, filtration and/or dilution. This makes sure the sample is ready for analysis. The sample is then introduced into a nebulizer – a glass device that transforms the liquid into a fine aerosol. The aerosol passes through a spray chamber, where oversized droplets are removed, before entering the inductively coupled plasma, the central component of the ICP-MS. If needed, the generated aerosol can be diluted with an extra stream of argon, as is the case with the ICPMS-2040 or -2050.
The plasma itself is generated by a high-frequency coil, which ionizes argon gas. Applying high-frequency energy and ionizing the argon gas means that extremely high temperatures of around 6,000–10,000 K can be reached. As shown in Figure 1, the aerosol particles of the sample are ionized in the plasma by stripping electrons from the atoms, resulting in the formation of positively charged ions.
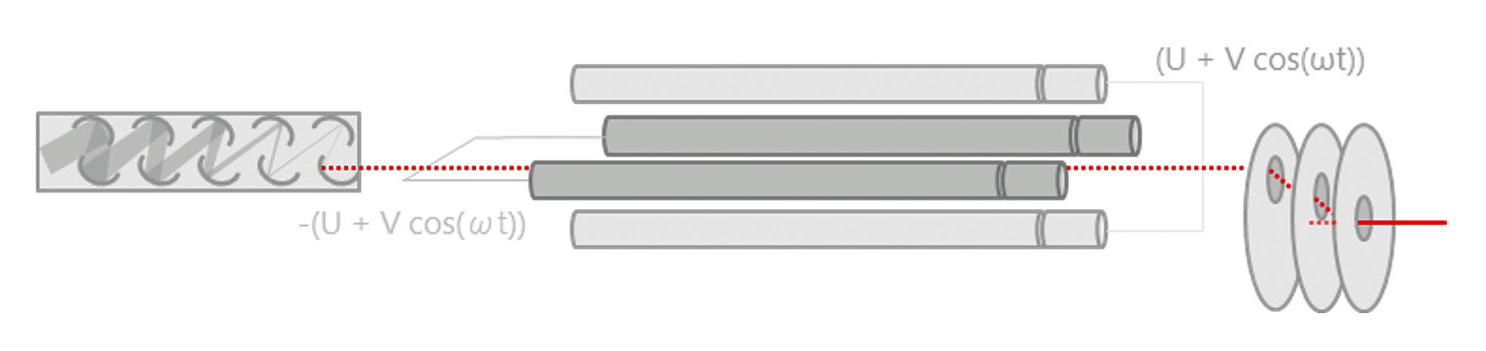
After various interferences are eliminated using collision or reaction gases and non-measurable neutral particles are removed via a lens system, the sample passes through a quadrupole mass filter. As depicted in Figure 2, this component separates ions according to their mass-to-charge ratio (m/z). Once the ions are separated by mass, they reach the detector, which is typically an electron multiplier. The detector counts the ions and measures the intensity of the signals generated by the different ions. This intensity is directly proportional to the concentration of each element in the sample. The data collected are analyzed to determine both the types and amounts of elements present in the sample.
How does ICP-MS outperform other methods?
Compared to traditional measurement techniques, ICP-MS provides huge benefits. It can analyze many elements at the same time, making the process more efficient. Thanks to its ability to detect over 70 different elements in a single sample, even complex wastewater samples can be thoroughly characterized. This is particularly important because wastewater frequently contains a wide range of pollutants that need to be monitored simultaneously.
The method is also impressively fast: The results are ready relatively quickly, which is essential for the timely monitoring of wastewater quality, especially in industrial settings where rapid decisions are needed. In addition, ICP-MS experiences lower matrix effects compared with other techniques, such as ICP-OES. This results in more accurate and reproducible outcomes, which is particularly important when analyzing wastewater samples that often contain complex chemical mixtures.
Analysis according to DIN EN ISO 17294-2
The Waste Water Ordinance permits ICP-MS in accordance with DIN EN ISO 17294 as an approved method for analyzing heavy metals, with the exception of titanium and mercury. Before ICP-MS analysis, nearly all target elements require the sample to undergo acid digestion in accordance with DIN 15587-2. This ensures that turbid or particulate-containing samples are properly and consistently prepared for analysis. As shown in Figure 3, this method makes it possible to create solutions ready for analysis even from sometimes very turbid samples. If the samples are not prepared in this way, the ICP-MS system’s tubing, capillaries and glass components could quickly become blocked, and cross-contamination might occur.
The DIN EN ISO 17294-2 standard, which governs the measurement of heavy metals using ICP-MS, defines the fundamental requirements in its key aspects. The table outlining the recommended isotopes for each element is especially helpful here. Other tables list the possible interferences and applicable correction formulas, which can be incorporated into the instrument software.
One example of this is the measurement of nickel at mass 58: The results could be affected by high iron concentrations, since iron – with an isotopic abundance of 0.282 % – also occurs at mass 58, resulting in an isobaric interference. For accurate results, automatic correction using a formula that accounts for the isotopic distribution is recommended.
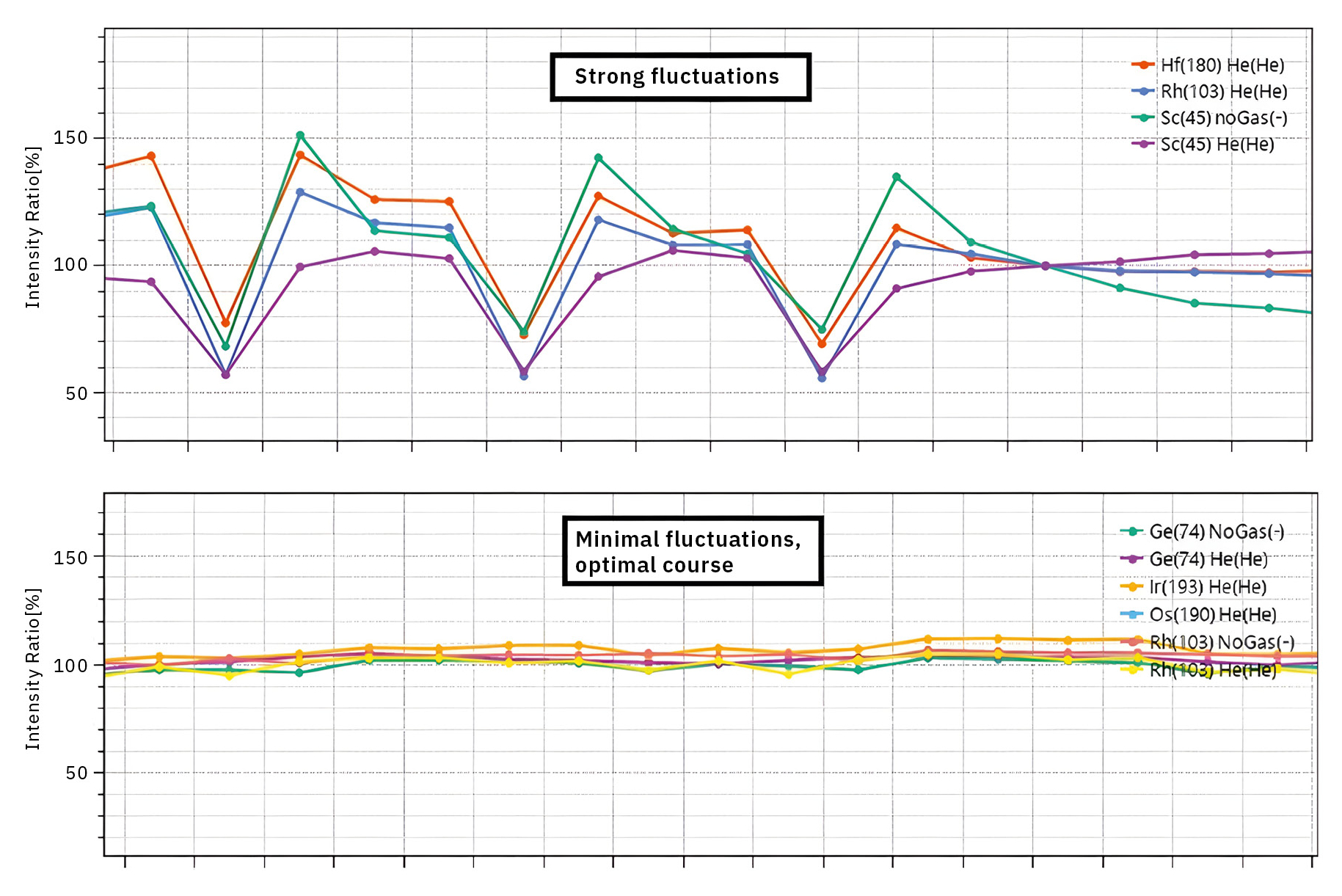
Specifying a maximum salt concentration of 2 g/L for the sample, corresponding to a maximum electrical conductivity of 2,700 µS/cm, is also advisable. According to the standard, this is done to prevent both spectral and physical interferences. If necessary, diluting highly concentrated samples serves as a type of matrix adjustment, or “matrix matching”, and enables long-term stable measurements, even across samples with differing levels of contamination. This is especially relevant for wastewater samples, which can sometimes contain very high concentrations of various macroelements from the alkali and alkaline earth groups, along with other, sometimes unidentified contaminants – mainly sodium, magnesium and calcium. Present in high concentrations, these elements can significantly weaken the plasma due to their chemical and physical properties, leading to reduced recovery of the internal standards. Figure 4 provides an example highlighting this difference.
Highly toxic mercury – an obvious candidate for ICP-MS
Mercury is one of the most critical trace elements due to its extreme toxicity, which can severely affect both humans and animals. It can have acute or chronic effects, primarily damaging the nervous system, kidneys and immune system. Unborn babies and young children are particularly at risk, as mercury can impair brain development and lead to long-term neurological damage.
The environmental persistence of mercury is a critical concern, since it persists in soils, water and the atmosphere and is difficult to break down, resulting in long-term ecosystem contamination. As well as this, mercury also accumulates in the food chain. Predatory fish, at the top of the chain and feeding on contaminated organisms, can contain substantial concentrations, posing a risk to humans who consume them.
The various sources of mercury in the environment, from industrial emissions to mining, waste incineration, and so on, make monitoring and regulation complex tasks. Precise analytical methods are required to reliably quantify mercury in water, soil and biological samples. Due to its toxicity, strict requirements are in place for the monitoring and reduction of mercury. Regulations, including the Substitute Building Materials Ordinance, already employ mercury analysis in accordance with DIN 17294. ICP-MS is particularly well suited for this, offering both the necessary sensitivity and robust performance.
Good to know: Since mercury is highly volatile and prone to sticking to glass surfaces and capillaries, it is recommended to stabilize samples, standards and rinse solutions by adding a small amount of gold. The added gold interacts with the mercury to form unstable amalgam complexes, which stabilizes the mercury content over time, preventing cross-contamination.
Sensitivity meets strength – many features for a tailor-made method
Wastewater samples are often highly concentrated and contain unknown contaminants. The challenge here is developing a suitable method that combines the required sensitivity with maximum strength. Ideally, both macro- and trace elements should be analyzed at the same time – over an extended period and with as many samples as possible in a short time. There are many “adjustable parameters” available to achieve this goal. Predefined methods (also called preset methods) in LabSolutions ICPMS can also be useful, since they include preset parameters tailored to the sample matrix, which enhance the strength of the procedure and protect the instrument.
Matrix effects can be minimized not only through conventional sample dilution but also by reducing the peristaltic pump speed, increasing the distance between the torch and cones and using argon dilution.
To optimize the measurement of specific elements in a sample, the integration time for each element can be adjusted: Depending on the required sensitivity, macroelements can be measured using shorter integration times, whereas trace elements typically take longer.
Features such as proactive rinsing and a compact autosampler further shorten measurement times, allowing samples to be analyzed in under two minutes – depending on factors such as the number of elements being measured.
Overall, by optimizing sample preparation, instrument settings and procedural workflows, it is possible to develop a strong, time-efficient method capable of reliably analyzing both macro- and trace elements in wastewater.

Getting to the point
Two factors work together to safeguard the environment and water quality: The German Waste Water Ordinance sets clear limits for the discharge of wastewater and requires wastewater treatment plant operators to conduct comprehensive monitoring and treatment. Chemical analysis, particularly methods such as ICP-MS, enables the precise identification and quantification of pollutants, including heavy metals and especially critical elements like mercury. These analytical methods are essential for complying with legal requirements and thereby minimizing potential risks to the environment and human health. And that’s why the continuous application and optimization of analytical methods will remain of central importance in the future.