Think big – purity on a large scale
Preparative HPLC as the key to isolating active ingredients
Dr. Martin Meyer, Shimadzu Europa GmbH
Medicines save lives, ease suffering and shape modern medicine in ways that almost no other achievement has. However, before an active ingredient can contribute to a cure, it must be developed, tested and produced with the highest levels of purity. This is due to the fact that effectiveness depends not only on the correct dosage and application but also on the purity of the ingredients. This is where preparative HPLC comes into play – a key technology that is decisive for the quality and efficacy of new medicines. This article will explore whether the technology is capable of meeting the strict requirements.
When developing drugs, researchers are working tirelessly to develop new medicines that make it possible to fight infections, manage chronic diseases and improve the quality of life of millions of people. These include passionate chemists, researching promising new active substances that might even have the potential to treat rare but devastating diseases.
That being said, isolating an active substance doesn’t come without challenges. The chemists have to deal with what is often a complex and demanding process in which there is always a risk that residues from the starting compound will remain in the end product or that unwanted by-products will be created.
These impurities could not only impair the effectiveness of the medicine itself but also cause toxic reactions or trigger unexpected side effects in the people taking them. A huge responsibility, since there’s a fine line between a cure and a poison.
With this in mind, it’s important that chemists use a process capable of efficiently isolating the main substance and separating the unwanted compounds at the same time. Various methods such as crystallization, filtration and distillation are at their disposal here.
However, a technique that not only separates the existing substances but also identifies them would be particularly valuable in the development process. High-performance liquid chromatography (HPLC) makes this possible. It provides chemists with new ways to precisely isolate and analyze their target compounds.
HPLC is an analytical technique that is widely used in the chemical and pharmaceutical industries. It allows complex mixtures to be separated and analyzed, which is essential for quality control and research.
Going from analytical to preparative HPLC
HPLC is usually optimized to be able to detect extremely small concentrations of substances. However, when it comes to isolating larger quantities of an active ingredient, it is necessary to select significantly larger dimensions. Both the device components and the method itself must be scalable here.

A critical component in determining how much of a substance can be applied to a chromatographic system is the separation column. Generally speaking, separation columns for analytical applications have an internal volume of 0.1 to 3 mL and can separate around 3–20 mg of substance. In contrast, preparative columns have an internal volume of 20 to 300 mL and can achieve separation capacities of 300 to 2,000 mg.
Due to the larger volume of the preparative columns, the flow rates of the liquid must also be adjusted, meaning that solvent consumption can be 20 to 100 times higher than in analytical applications.
For this reason, it’s important to carry out preparative HPLC ideally in combination with already established methods in order to minimize solvent consumption.
As most experienced analysts know, method development is one of the most time-consuming parts of analytics. This is why it’s better to first develop the method for separating the active ingredient from by-products on an analytical scale and then scale it up to a preparative scale. This scaling process is relatively straightforward and can be fully automated using software from Shimadzu (Figure 1).
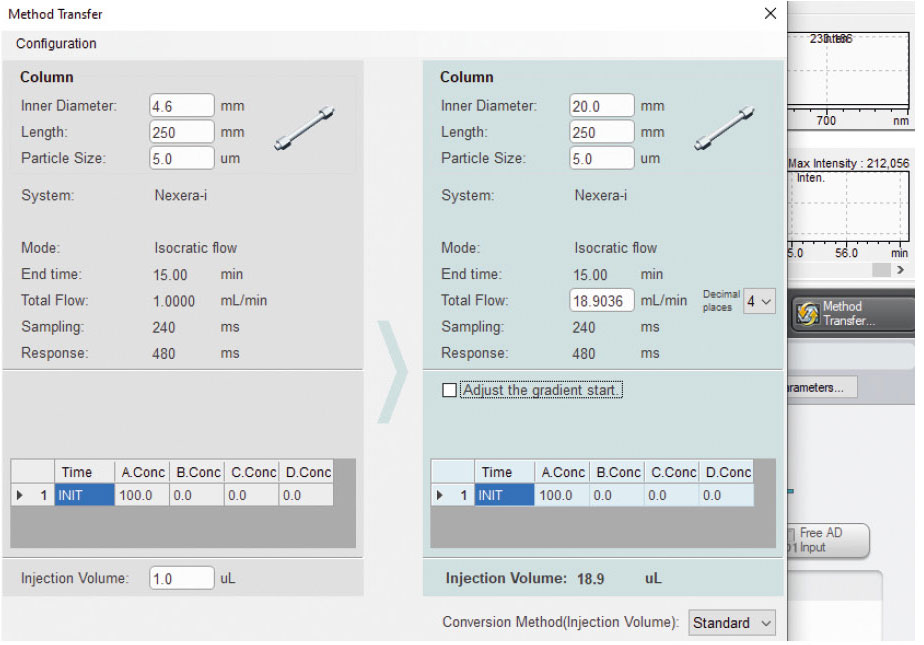
For upscaling to work successfully, it is essential that both the analytical and preparative columns are very similar in terms of their separation behavior. Using the same series of columns is therefore recommended.
With the Shim-pack G and the Shim-pack Scepter series, Shimadzu is offering two column ranges that are fully available from small analytical dimensions to large preparative columns (Figure 2).

fdfd
fd
fdfd
fd

Going from salicylic acid to Aspirin®
Upscaling and isolation using preparative HPLC can be demonstrated using the example of one of the world’s best-known active ingredients: acetylsalicylic acid (ASA) – Aspirin®.
ASA is an analgesic, anti-inflammatory, antipyretic and antiplatelet (TAH) drug from the group of non-steroidal anti-inflammatory drugs (NSAIDs) and has been marketed by pharmaceutical manufacturers in various products since the beginning of the 20th century. The active ingredient is also on the list of essential medicines published by the WHO.
The starting material for ASA is salicylic acid, which comes from a variety of plants. However, salicylic acid is not as well tolerated, is less effective and is poorly absorbed by the body compared to Aspirin® (acetylsalicylic acid). Aspirin® is synthesized by the acetylation of salicylic acid with acetic anhydride.
Nevertheless, it’s possible for residues of salicylic acid to remain in the end product during synthesis. These unwanted residues can be effectively separated using preparative HPLC, which guarantees the purity of the end product (Figure 3).
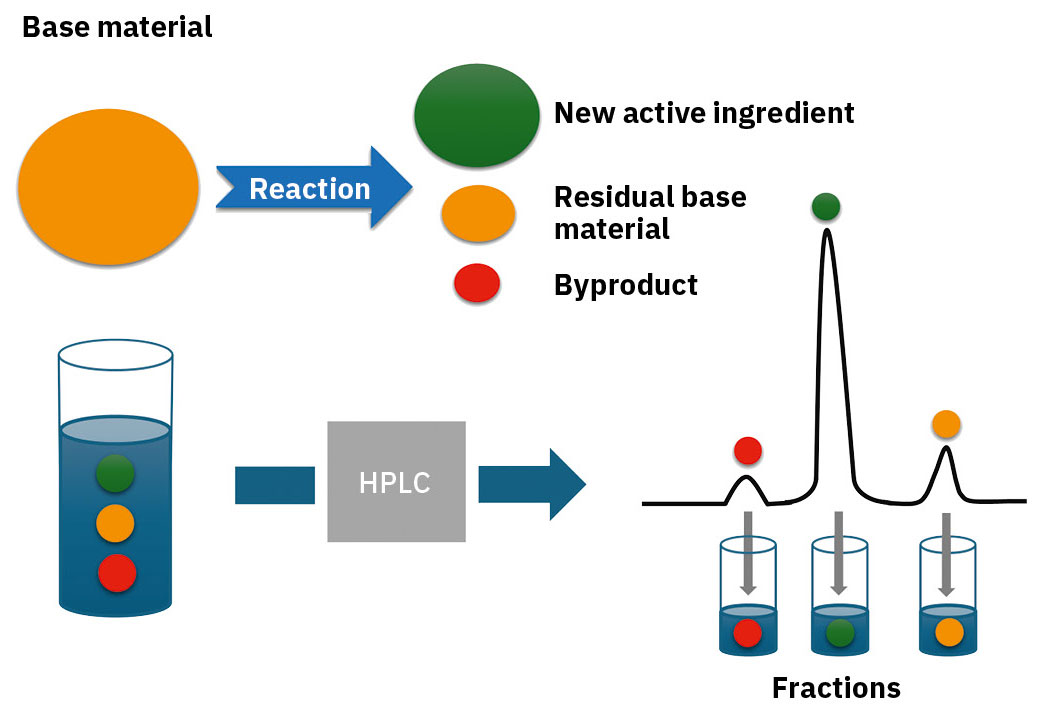
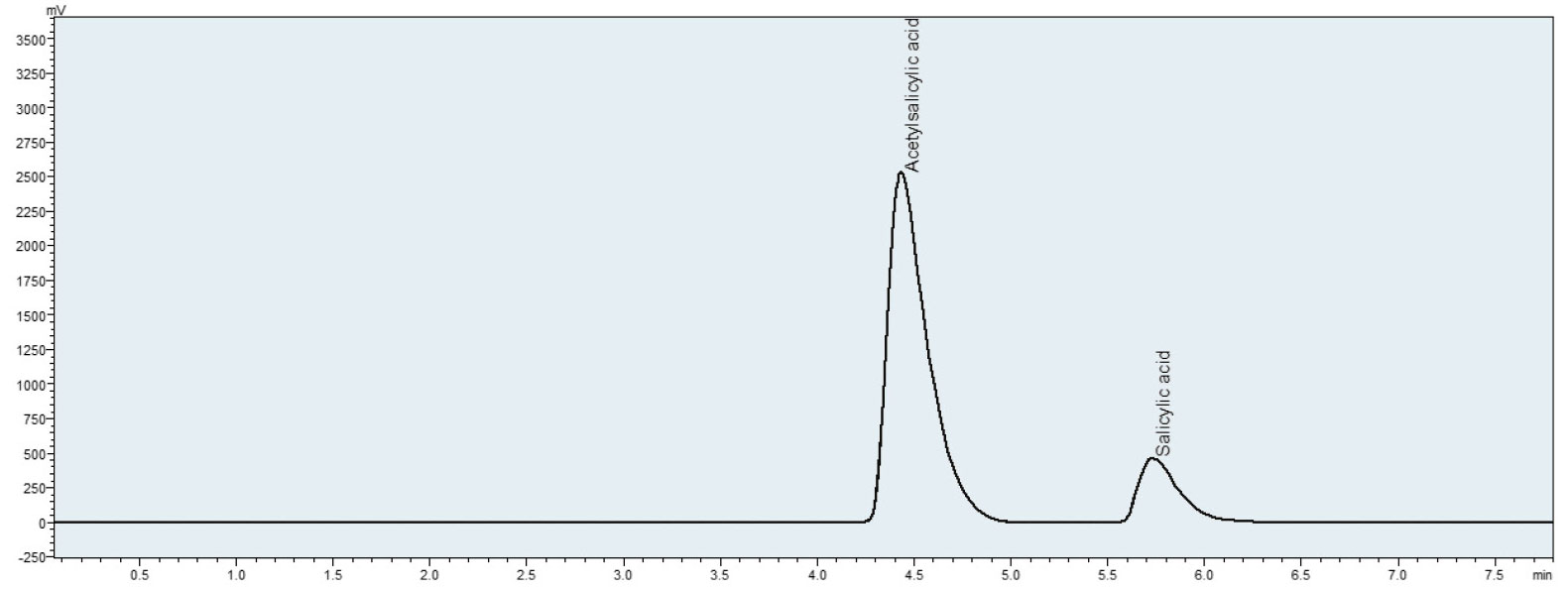
To demonstrate this, a method was developed to establish the retention times for Aspirin® and salicylic acid through the use of standards (analytical parameters can be found in Table 1). Next, a sample of the synthesized Aspirin® was examined. The findings show the presence of salicylic acid next to the main product (Figure 4).
| Analytical method | |
| System | Nexera Prep/LH-40 |
| Column | Shim-Pack GIST 5 µm 250 x 4.6 mm |
| Mobile Phase | Water 50%/Acetonitrile 50 %/ 0.5 % phosphoric acid |
| Program | Isocratic |
| Run time | 10 min |
| Flow rate | 1 mL/min |
| Injection time | 1 µL |
| Detector | SPD-40 (standard cell) 200 nm |
The parameters were then transferred to the preparative dimensions (Table 2). A measurement was carried out with this method to ensure that both methods yield similar results.
| Preparative method | |
| System | Nexera Prep/LH-40 |
| Column | Shim-Pack GIST 5 µm 250 x 20 mm |
| Mobile Phase | Water 50%/Acetonitrile 50 %/ 0.5 % phosphoric acid |
| Program | Isocratic |
| Run time | 10 min |
| Flow rate | 19 mL/min |
| Injection time | 20 µl/1,000 µL |
| Detector | SPD-M40 (prep cell) 200 nm |
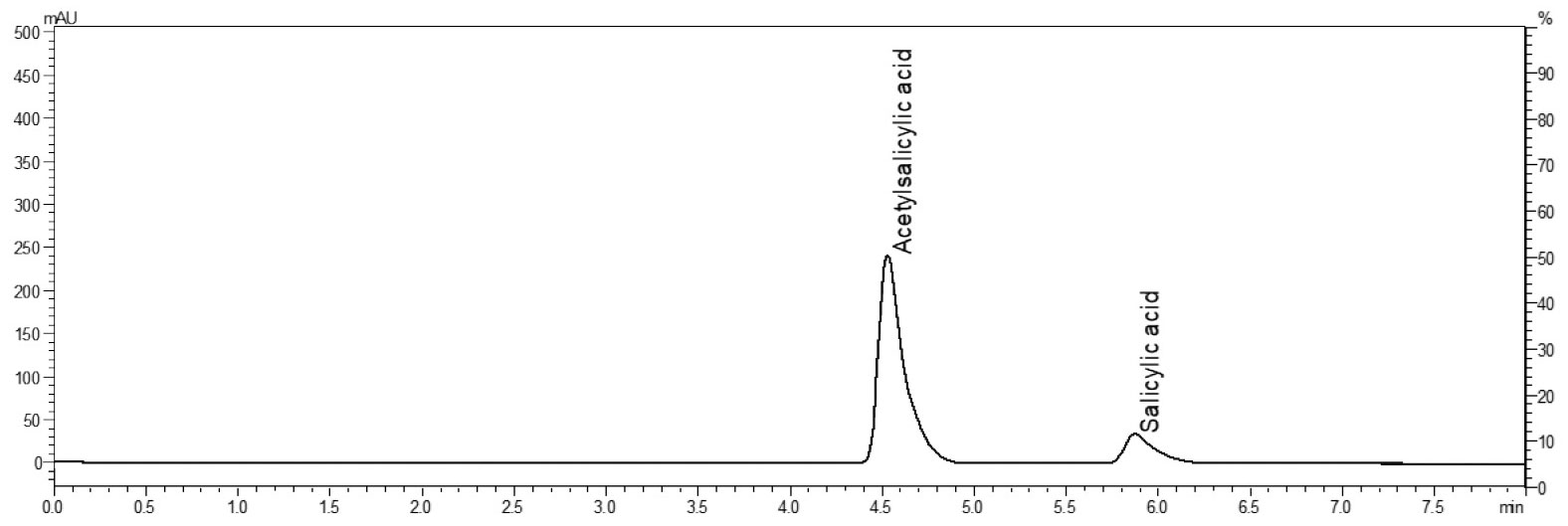
Although the column dimensions are substantially different and the flow rate was increased by 20 mL, separation remained almost identical. Based on this verification, the fractionation was set so that the system starts collecting the compounds at the right times (Figure 6). On top of this, the amount of material was greatly increased to ensure maximum yield of the substances.
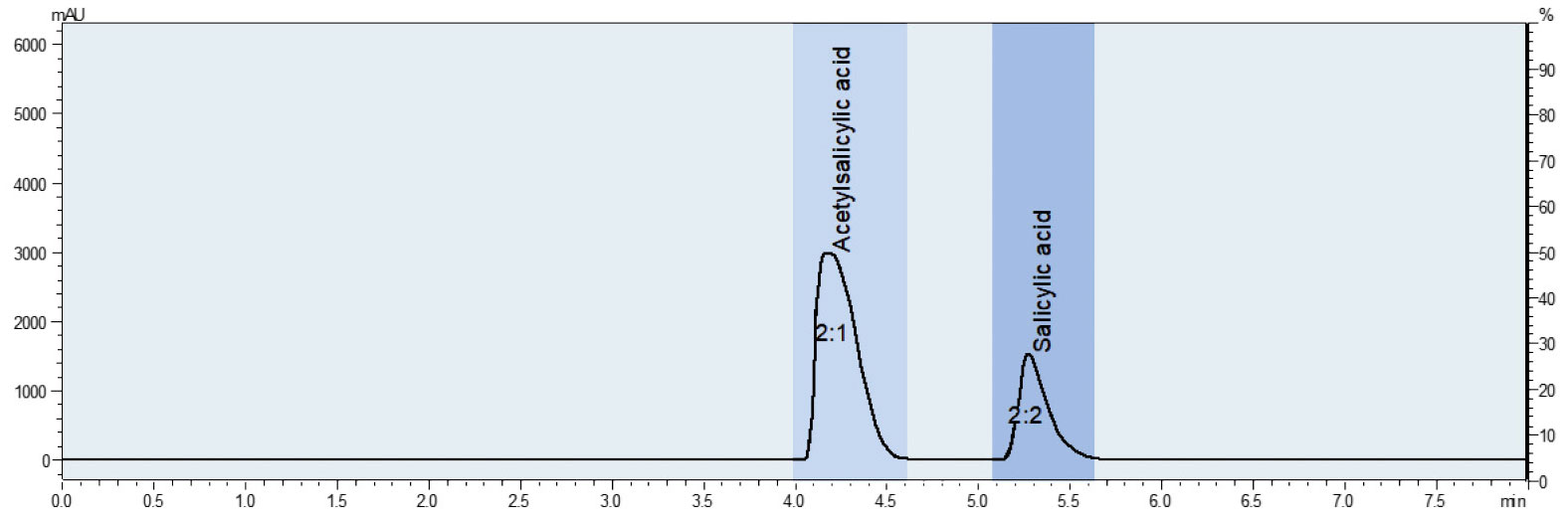
The fractions obtained can be tested for purity in another step. As shown in Figure 7, salicylic acid is no longer detectable in fraction 1, indicating that the active ingredient has been successfully isolated.
However, preparative liquid chromatography is not only used in the pharmaceutical sector but also plays a key role in the food industry, biotechnology, natural product chemistry and the cosmetics industry in that it contributes to the isolation and purification of valuable compounds.
Versatile technology for better patient safety
Scaling up analytical HPLC columns to preparative HPLC columns is an essential step in chemical synthesis and analysis. The synthesis of Aspirin® from salicylic acid was used to demonstrate how preparative HPLC from Shimadzu effectively removes impurities and produces a pure product that guarantees patient safety. This technique is not only relevant for Aspirin® synthesis but can also be applied to numerous other chemical compounds, which highlights its versatility and importance in a number of industrial sectors.

